Application of 3A Zeolite Prepared from Venezuelan Kaolin for Removal of Pb (II) from Wastewater and Its Determination by Flame Atomic Absorption Spectrometry ()
1. Introduction
The contamination of wastewater by toxic substances is a worldwide environmental problem causing in organisms numerous diseases and disorders [1,2]. Pollutants present strong toxicity at weak concentrations, even when they are at trace levels, such as heavy metals [3]. Heavy metals ions are of great concern, due to their mobility in natural water ecosystems and that, unlike organic pollutant, heavy metals ions do not undergo biological degradation and tend to accumulate in the organisms, thereby eventually entering the food chains [2]. The environmental protection forces as the World Health Organization, WHO [4] and the Decree 833 in Venezuela [5] limit the contents of lead in drinking water between 10 and 50 μg·L−1 respectively and wastewater in 0.5 mg·L−1. Therefore, the removal of heavy metals from industrial effluents is very important and the proper treatment of this effluent is crucial for a viable industry [4,5]. Various techniques such as chemical precipitation, electroflotation, ion-exchange, membrane separation, reverse osmosis, electrodialysis and solvent extraction have been investigated for the treatment of effluents containing heavy metals, but these methods are expensive which increases the cost of water treatment [6,7]. Therefore, there is a demand for a treatment strategy that is simple, robust, and which addresses local sources and regulations [4]. Ion exchange is feasible when an exchanger has a high selectively for the metal to be removed and the concentrations of competing ions are low. The metal may then be recovered by incinerating the metal-saturated resin and/or to with solvents for extraction, however, the cost of such processes naturally limits its application to only the more valuable metals [8]. Also in many cases, the heavy metals are not valuable enough to warrant the use of special selective exchangers/resins from an economic point of view. This has encouraged research into using low-cost adsorbent materials to purify water contaminated with metals. [8]. Many adsorbents have been investigated for ions removal from water and environmental samples, for example, activated carbon [9,10], silica [11,12], fullerene [13] and zeolite [7,8,14]. Zeolite is an aluminum silicate that occurs both as natural and as produced synthetic [15] and they belong to the class of mineral known as “tectosilicates” [1]. The structures of the zeolites consist of three-dimensional frameworks of SiO4 and AlO4 tetrahedra with pores. The aluminum ion is small enough to occupy the position in the center of the tetrahedron of four oxygen atoms, and the isomorphous replacement of Si4+ by Al3+ produces a negative charge in the lattice [1,16]. The net negative charge is balanced by the exchangeable cation such as sodium, potassium, calcium or proton, a counterion that is present in the pores. The cations are exchangeable with certain cations in solutions such as Pb, Cd, Zn and Mn [16], which can be easily regenerated [4]. The cationic exchange property is a function of the radio of Si to Al. This capacity is expressed as the number of cations per mass or volume unit available for exchange. The A zeolite is usually synthesized with sodium as the changeable cation [17] and the chemical composition of that zeolite is represented by Na12Al12O48 × 27H2O [18]. Due to the position of the cations in the zeolite structure, the effective pore diameter may vary according to the type of compensation cation. In the case of a potassium (K+) cation, the effective diameter of the pore is approximately 3 Å and the zeolite is known as 3A. If the cation is sodium (Na+), the opening is 4 Å, and the material is referred to as 4A zeolite [17]. The A zeolite structurally is composed of sodalite cages, connected through double four-membered rings (D4R) of (SiO4)4− and (AlO4)5−. By this connection, three cages are present: D4R, sodalite cage, and α-cage. An eight-member oxygen ring defines the pore diameter between 0.23 and 0.42 nm [18]. Zeolites have wide applications as gas and odor filter, as a part of animal feed, as ammonia removers from different wastewaters, chemical sieve, water softener and adsorbents [7,8]. The objective of the present study is to investigate the performance and capacity of 3A zeolite prepared from Venezuelan kaolin, in the removal of lead from aqueous solution and wastewater and its determination by FAAS. We introduce kaolin mineral as a source material to prepare pure, single phase, highly crystalline 4A zeolite by hydrothermal method (750˚C) and shorter conversion time, and then the exchange of sodium to potassium in reflux system by temperature controlled. In particular, the influence of experimental conditions such as pH, lead ion concentration, and time was explored under batch conditions. The Langmuir and Freundlich models were used to describe the interaction between the metal ion and the adsorbent. Knowledge on this topic could be useful in designing wastewater treatment systems using 3A zeolite as a low-cost adsorbent.
2. Experimental
2.1. Chemical and Solutions
All reagents were of analytical reagent grade and deionized water was used throughout. Working standard solutions were prepared freshly at various concentrations by diluting the stock solution of 1000 mg·L−1 lead (II) ion (Merck, Darmstadt, Germany) with water that was distilled/deionized and further purified using a Milli-Q water system (18 MΩ cm, Millipore, Bedford, MA, USA). HNO3 (65% v/v, Merck, Darmstadt, Germany) were used for samples acidify. Venezuelan kaolin was used in the synthesis of the zeolite and NaOH (98%, Merck, Darmstadt, Germany). The kaolin was collected from deposits located specifically at a place known as Km 88 (China Clay Guayana C. A.) from Bolivar City (see Figure 1), looking like a white powder, with particle size less than 200 mesh. A solution of 1.00 mol·L−1 of KCl (99%, Merck, Darmstadt, Germany) prepared by dissolving the salt was used for exchange. Wastewater samples were obtained from Lake Valencia, Valencia City and filtered through Whatman filter paper (No. 42), acidified with 0.03 mol·L−1 HNO3 and stored at (4.0˚C ± 0.1˚C) in acidcleaned polyethylene bottles in order to determine the “dissolved metal” fraction. All bottles used for storing samples as well as the glassware were washed in 10% v/v HNO3 for 24 h and finally rinsed with ultrapure water.
2.2. Apparatus
Atomic Absorption Spectrometry
Perkin-Elmer Model 5100 PC flame atomic absorption spectrometer (Norwalk, CT, USA) was used as the detec-
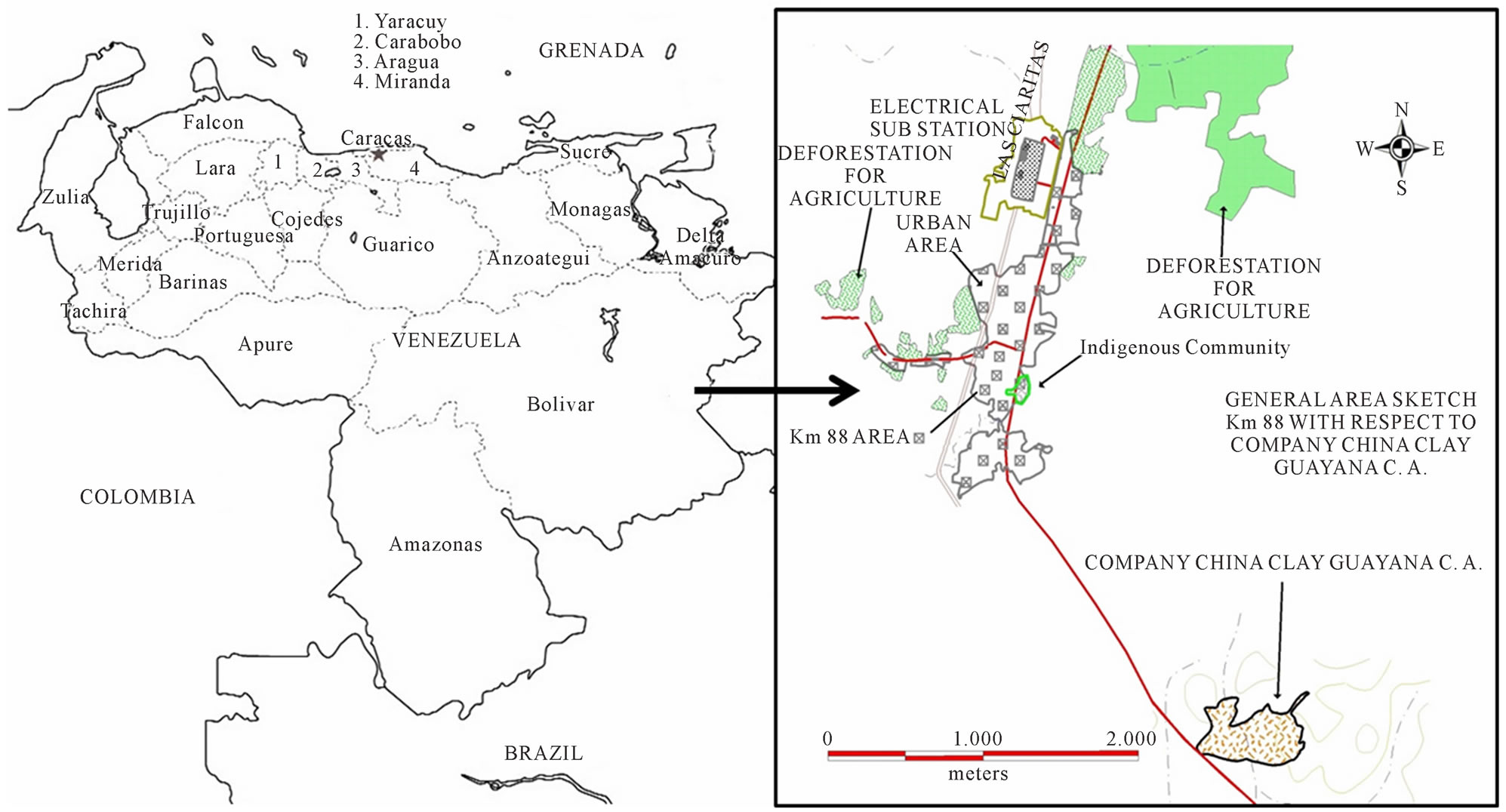
Figure 1. Sampling location of the Venezuelan kaolin, Km 88, company China Clay Guayana C. A.
tion system. A Perkin Elmer IntensitronTM hollow cathode lamp for lead, operated at 10 mA was used as the light source and a deuterium lamp as background corrector was employed. The wavelength/monochromator spectral bandpass (slit) was set at 217/0.7 nm. The spectrometric measurement was carried out using an air/acetylene flame at 7.6/2.6 L·min−1 flow rate, according to the manufacturer recommendations. Under these conditions, the nebulizer’s free uptake rate was 6.5 mL·min−1. Peak height was used for signal evaluation throughout the study. The pH values were controlled by a Professional Benchtop pH meter BP 3001 (Itrans Instrument, Singapore).
2.3. Characterization of Zeolite
All prepared zeolites were characterizes by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy-energy dispersive X-Ray (SEM-EDX) and X-ray diffraction (XRD). FTIR was performed using a FTIR Perkin Elmer (Germany), Spectrom RX 1. The zeolite samples for the infrared studies were recorded in potassium bromide disk. For the micrographs was used a scanning electron microscope Hitachi S-2500 (Tokyo, Japan) coupled to an energy dispersive X-ray microanalyser Thermo Noran for the elemental analysis. XRD was performed using a Bruker D 8 Focus powder diffractometer (Karlsruhe, Germany) equipped with an X-ray tube (Cu-Kα radiation). A small quantity of the sample was ground mechanically in an agate mortar and pestle and mounted on a flat holder covered with a thin layer of grease. Data collection was carried out in the 2θ range 2 - 70˚, in steps of 0.02˚ and counting time of 24.5 s. Phase identification was performed by searching the ICDD powder diffraction file database, with the help of JCPDS (Joint Committee on Powder Diffraction Standards) files for inorganic compounds [19]. The relative intensity yields were obtained from normalized XRD intensities of the major reflection for each material.
2.3.1. Preparation of 4A Zeolite for Laboratory Experiment
The methodology used to synthesize zeolite A is based on the work of Imbert et al. [20]. The raw material for the synthesis used in this study was Venezuelan kaolin. Kaolin (Al2O3 × 2SiO2 × 2H2O) was subjected to dehydroxylation process to produce a metakaolin (Al2Si2O7) and then was zeolitized, producing 4A zeolite. The process involved the calcination of the kaolin at (750.0˚C ± 0.1˚C) for 5 h, in a muffle furnace Heraeus Electronic (Dreieich, Germany). The metakaolin formed is more reactive than the kaolin, and the nonstructural minerals can be oxidized. Control over the calcining temperature and time is very important for the desired zeolite type. Higher temperatures generally lead to the production of denser phase while cooler temperatures produce more open structures [20]. The precursor gel was prepared by mixing 8.00 g de kaolin previously calcined with 120 mL de NaOH 2.00 mol·L−1. The synthesis was performed by adding the precursor gel in a Teflon reactor coupled with a water bath set at (25.0˚C ± 0.1˚C) and after stirred (100 rpm) for 1/2 h (digestion process) in a Vibratory Mill type MM-2 with time control and speed (Retsch, West Germany), for mixing efficiently the precursor gel in the process of the zeolitization. The crystallization process was realized by a resting at (80.0˚C ± 0.1˚C) for 24 h. The resulting material was washed five times with distilled water (pH about 8), centrifuged, and dried in the stove Thermo Scientific (Iowa, USA) at (120.0˚C ± 0.1˚C) overnight.
2.3.2. Obtaining 3A Zeolite through Ionic Exchange
The synthesis of 3A zeolite was made through ionic exchange with the 4A zeolite. The changeable cation of the 4A zeolite is sodium, and in this case potassium chloride was used in the ionic exchange. The methodology employed was a reflux system with controlled temperature where 4.00 g of 4A zeolite was added to a bottle of 250 mL contained 120 mL de KCl 1.00 mol·L−1.
The mix was allowed in reflux to (80.0˚C ± 0.1˚C) for 4 h. The resulting material was washed with distilled water and checked the presence of chlorides with AgNO3 0.05 mol·L−1. The crystals dried at 120.0˚C ± 0.1˚C overnight on the stove.
2.4. Batch Sorption Studies
Batch sorption experiments were carried out at mix 50 mL jars at ambient temperature (21.0˚C ± 0.1˚C) that contained 70.00 mg of 3A zeolite and 25 mL of 50 μg·mL−1 of Pb2+ ions, which were stirred for 1 h. The mixtures were centrifuged and then the supernatants filtrated. Also, the parameters (following the univariate method) such as the dosage of zeolite and period of shaking were optimized. The final concentration of metal (equilibrium) was determined by FAAS under triplicate measures. The percentage of metal removal adsorption % R and the amount of metal ions adsorbed per gram of adsorbent at equilibrium qe (mg·g−1), were calculated using the equations:
 (1)
(1)
 (2)
(2)
where C0 is the initial metal concentration and Ce is the metal concentration after contact with the zeolite (mg·L−1), V is the volume of the solution (L) and m is the weight of the adsorbent (mg) [1,7].
3. Results and Discussion
3.1. Characterization of Sorbent
Based on the chemical analysis, it is possible to calculate the Si/Al ratio of the kaolin, and of the obtained zeolites. The Si/Al proportions were 1.00 for the kaolin, 1.17 and 1.20 for the 4A and 3A zeolites respectively, whose expected approximate value is 1 for the A zeolite [16]. The kaolin has little potassium (<1.00%), which is ideal for obtained zeolites. The SEM pictures (Figure 2) show kaolin exfoliated into thin slices (layers), the 4A zeolite and 3A zeolite crystallizes in fine particles, presenting a greater amount of well-formed cubic crystals. The X-ray diffractogram of the 3A zeolite can be observed in Figure 3, which presents high crystallinity. The plot shows all the characteristic peaks matching with the reported in the diffraction equipment library, with corresponding formula to K12Al12Si12O48 × 20H2O (Ref. Code 00-070 - 1878) [19]. Also, a small amount of quartz SiO2 that is very common (Ref. Code 01-079-1906) [19] is identified. The infrared spectra of kaolin, 4A zeolite and 3A zeolite are shown in Figure 4. The transformation of kaolin to 4A zeolite can be clearly observed from IR spectra in the lattice region (1100 - 450 cm−1). The kaolin starting material gives well-defined IR bands in this region due to Si-O, Si-O-Al, and Al-OH vibrations. The

Figure 2. SEM micrographs of: (A) kaolin, (B) 4A zeolite and (C) 3A zeolite.

Figure 3. X-ray powder diffraction patterns of 3A zeolite.

Figure 4. FTIR spectrum of kaolin and synthetized zeolites.
conversion to 4A zeolite totally removes these bands, leaving a broad intense asymmetric band between 1050 - 980 cm−1 as the major feature. Moreover, it was observed the disappearance of the band at 787 cm−1, indicating the loss of Si-O-Al units; this is consistent with distortion of the tetrahedral and octahedral layers. The band for the zeolite framework at 500 cm−1 is due to vibration of the double ring of four tetrahedral (D4R), which is dominant in the secondary building unit of the structure of the type A zeolite. The bands at 3700 - 3400 cm−1 are known to assignable to Si-OH, Si-OH-Al and -OH hydroxyl groups. The vibration and flexion bands found in the 3A zeolite spectrum are similar to those found it in the 4A zeolite spectrum, it just appear a lightly shift of the bands in the 400 cm−1 zone, due to the ionic exchange.
3.2. Analytical Features
The method was evaluated through the main analytical features, shown in Table 1. The LOD obtained from CLOD = KbSbm−1 for a numerical factor Kb = 3 and “Sb” is the standard deviation of the blank solution (n = 6) and “m” is the slope of calibration curve [21]. A satisfactory correlation was obtained (R = 1) in the linear range studied. The repeatability of the proposed method was assessed by performing five consecutive of metal removal at a concentration level of 50 mg·L−1, and expressing the result in terms of the relative standard deviation. A value of 1.4% was obtained, demonstrating an excellent repeatability.
3.3. Influence of pH on Pb (II) Adsorption
The process of metal ion adsorption by zeolites is significantly affected by the pH. It is reported that H+ ions are more successfully adsorbed by zeolite rather than metal ions [6]. The effect of pH on the adsorption the Pb2+ ions was investigated in the range of 2 - 8, using 50 mg of zeolite contacted with 25 mL solution of 50 mg·L−1 of Pb2+ ions, with continuous agitation at a speed of 1500 rpm at ambient temperature (21.0˚C ± 0.1˚C) for 2 h. The results are shown in the Figure 5. Firstly we observe that at low pH values (pH < 4), the trend is the zeolite hydrolysis

and lead ions tend to be little retained by the zeolite. The

Table 1. Analytical features for the determination of lead by the proposed method.

Figure 5. Influence of pH on the retention of Pb2+ ions with 3A zeolite.
zeolites preferentially adsorbs H+ ions from solution to heavy metal ions, and thus in more acidic conditions more H+ ions are adsorbed from solution [6]. Secondly, with increasing pH, the competition from the hydrogen ions decreases and the positively charged Pb (II) ions can be adsorbed at the negatively charged sites on the adsorbent. Under these circumstances, the adsorbing cation might be species such as Pb2+, Pb(OH)+, Pb(OH)2,  ,
,  [22]. At pH lower than 7.5, Pb (II) ions were the dominant species, Pb(OH)2 was present at pH higher than 7.5 (pkps = 14.4).The removal optima was at a pH value of 6.5.
[22]. At pH lower than 7.5, Pb (II) ions were the dominant species, Pb(OH)2 was present at pH higher than 7.5 (pkps = 14.4).The removal optima was at a pH value of 6.5.
3.4. Effect of Adsorbent Dosage
The effects of different zeolite dosages on metal ion removal are illustrated in Figure 6, the adsorbent dosage varied from 30 to 150 mg/25 mL. The initial Pb2+ concentration, stirrer speed, initial pH and temperature were 50 mg·L−1, 1500 rpm, 6.5 and (21.0˚C ± 0.1˚C), respectively. It is observed from Figure 6, that the removal efficiency of adsorbents generally improved with increasing the amount of 3A zeolite. This was expected because the higher dose of adsorbent in the solution, the greater availability of exchangeable sites for the ions. Based on these results, 70 mg was considered as the optimum dosage with a removal efficiency of 78.8%.
3.5. Effect of Contact Time
The influence of agitation time on the removal percentage of metal ions was studied using a constant concentration of Pb (II) solution at (21.0˚C ± 0.1˚C) to determine the optimum contact time, using 3A zeolite. The applied time intervals were 15, 30, 45, 60, 90, 120, 180 min and another point after 24 h was taken in consideration in order to study the removal after a long time. Figure 7 depicts these findings. As shown, by increasing the contact time, the removal percentage increases until the adsorption equilibrium is reached. Furthermore, it also illustrates that the metal ion removal was fast in the first 60 min with 80.80% removal, which can be attributed to

Figure 6. Effect of the mass of adsorbent on the removal percent of Pb2+.

Figure 7. Effect of contact time on the removal of Pb2+.
the availability of sites for the Pb ions. The removal percent after 24 h shows a slight increase percent removal (6.5% more than removal percent after 60 min).
3.6. Effect of the Initial Metal Concentration
Samples (25 mL) containing 20, 30, 35, 40, 50, 60, 70 mg·L−1 of Pb2+ were dispensed in 50 mL Erlenmeyers flaks separately. To each of them, 70 mg of the synthesized 3A zeolite was added. The recipients were mechanically stirred for 1 h and then, the solutions were filtered off and their Pb concentrations determined by AAS. The results are shown in Figure 8. The removal efficiency percentage for Pb2+ decreased with increasing metal concentration in the aqueous solutions. These results indicate that energetically less favorable sites became involved with increasing metal concentration in the aqueous solution. During the ion exchange process, the potassium ions in the zeolitic structure enter to the solution, and Pb2+ ions from the solution bind to the zeolite. The lead ion exchange on the 3A zeolite can be exemplified by the equilibrium:
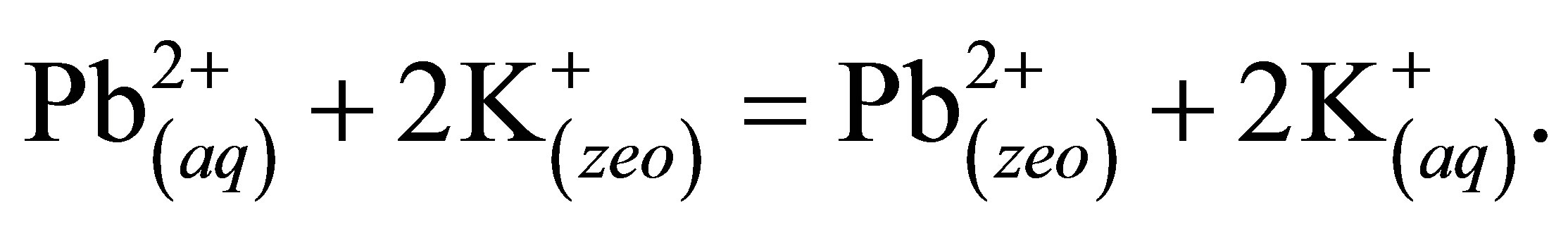
The maximum retention of the 3A zeolite was found to be 20 mg·L−1 (99.5% removal). In general, the decrease in the removal capacity of the zeolite material with

Figure 8. Effect of initial metal concentration on the % retention Pb2+.
increasing initial ion concentration may be due to higher probability of collision between the investigated ion and the zeolite particles. The variation in the percentage of removal may also be due to the fact that initially all sites on the surface of 3A zeolite were vacant and the metal ion concentration gradient was relatively high. Consequently, the extent of each ion uptake decreases significantly with the increase of contact time, depending on the decrease in the number of vacant sites or acid sites on the surface of zeolite material.
3.7. Desorption Studies
The regeneration ability of adsorbents for reuse is an important factor to assess their potential for industrial scale applications [7]. To be useful in metal ion recycling processes and/or half-life of the 3A zeolite, the metal ions should be easily eluted under suitable conditions. Elution of the Pb2+ ions from the zeolite was performed with 25 mL 0.1 N of selected desorption agents H2SO4, HCl, HNO3, NaOH, and acetone/HNO3, for 60 min. The Figure 9 shows the results, from which, it can be seen that HCl was the most efficient desorbing agent (can recover more than 72% of lead).
3.8. Removing of Lead Ions from Wastewater Samples
The application of the removing of lead from wastewater (real samples) was examined. These samples were filtered to remove suspended particulate matter. Four aliquots (25 mL) of the wastewater of Lake Valencia were analyzed for lead content by atomic absorption spectrophotometer prior to use. The wastewater samples had a very low lead concentration and therefore, they were spiked with 20 mg·L−1 of Pb2+, placed in a 50 mL Erlenmeyer flask and agitated at 1500 rpm, pH adjusted to 6.5 for 60 min at a temperature of (21.0˚C ± 0.1˚C). Then, extraction of lead from the four samples were carried out with a volume of 25 mL HCl 0.1 N for 60 min

Figure 9. Desorption of Pb2+ from 3A zeolite by various chemical agents.
and determined by atomic absorption spectroscopy to identify the percentage recovery values. Extractions were carried out on the basis of duplicate analysis. The results are given in Table 2, indicating the suitability of the 3A zeolite for the removal of lead ions. The permissible limits for lead in discharges to water bodies (effluent discharge) are of 0.5 mg·L−1, according to the Official Standards for water quality Venezuela 1995 (Decree 883, section III, article 10) [5]. It is obvious that 70.0 mg (optimum dosage) of 3A zeolite can reduce the Pb in residual water under the conditions mentioned.
3.9. Determination of Adsorption Isotherms
Sorption isotherms or capacity studies are of fundamental importance in the design of sorption systems, as they indicate how the metal ions are partitioned between the sorbent and liquid phases at equilibrium as a function of increasing metal concentration [22].
Several isotherm models are available to describe the equilibrium sorption distribution, namely, Langmuir, Freundlich and Dubinin-Radushkevich (D-R) [1], are the most used to describe sorption equilibrium for wastewater treatment applications [4]. The Langmuir isotherm model suggests that sorption of metal ions occurs uniformly by mono-layer adsorption on a homogeneous surface, with no interaction between the adsorbent ions [7]. It is represented as:
 (3)
(3)
where qm (mg·g−1) is the amount of adsorbate at complete monolayer coverage and b (L·g−1) is a constant relating to the heat of adsorption (the Langmuir constants) [8]. The Freundlich sorption isotherm, gives an empirical expression based on a heterogeneous system and a multilayer sorption [7], which assumes, the relationship between sorbate concentration at equilibrium and the exponential distribution of active sites and their energies [1]. The equation is commonly written as follows:
 (4)
(4)
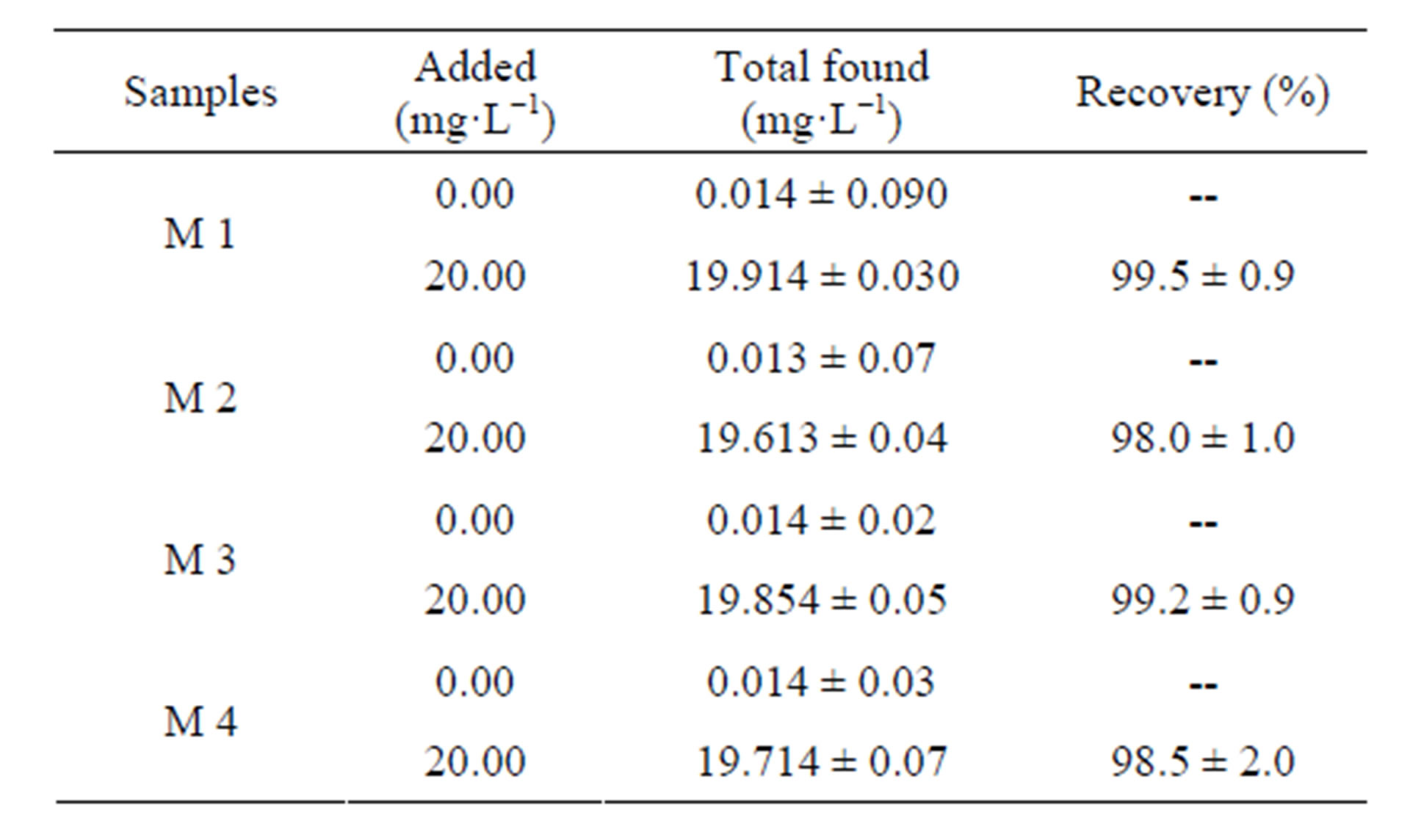
Table 2. Analysis of lead in different real samples.
where, Kf (mg·g−1) and m = 1/n are Freundlich isotherm constant, related to the sorption capacity of the sorbent and the energy heterogeneity of the system (and the size of the adsorbed molecule) respectively [22]. If the value of 1/n = 0, the isotherm is irreversible; 1/n > 1, the isotherm is unfavorable; 0 < 1/n < 1, the isotherm is favorable [7]. After mathematical transformation, the Equations (5) and (6) can be written as follows:
 (5)
(5)
 (6)
(6)
Another adsorption isotherm, the Dubinin-Radushkevich isotherm (D-R isotherm) was calculated from the adsorption data. This isotherm is more general than the Langmuir isotherm as it does not assume a homogenous surface or constant sorption potential. The D-R equation is expressed as follows:
 (7)
(7)
where β (mol2·J−2) is the activity coefficient related to mean sorption energy, and ε is the Polanyi potential, which is equal to:
 (8)
(8)
where R is the gas constant (kJ·mol−1·K−1) and T is the absolute temperature (K) [1,23,24]. The saturation limit Qm may represent the total specific micropore volume of the sorbent. The sorption potential is independent of the temperature but varies according to the nature of sorbent and sorbate. The slope of the plot of lnqe versus ε gives β and the intercept yields the sorption capacity Qm. The mean free energy of sorption changes when one mole of ion is transferred to the surface of 3A zeolite from infinity in the solution, and it is calculated from:
 (9)
(9)
The magnitude of E can be related to the reaction mechanism. If E is in the range of 8 - 16 kJ·mol−1, sorption is governed by ion exchange. In the case of E < 8.0 kJ·mol−1, physical forces may affect the sorption mechanism [24]. From the measurements of the isotherm studies, Pb2+ solutions (25 mL) of varying concentrations (20, 30, 35, 40, 50, 60, 70 mg·L−1) were agitated with 70 mg 3A zeolite conducted at (21.0˚C ± 0.1˚C) and pH of 6.5 and allowed to equilibrium for 60 min. After, aliquots of supernatants were withdrawn and the amount of the metal ion retained in the zeolite phase (mg·g−1) was estimated for AAS. All batch experiments were carried out in triplicated and the mean values are presented. Figure 10 shows the relationship between the amount of lead adsorbed per unit mass of 3A zeolite and its final concentration in the solution. Clearly, it indicates that the sorption capacity, initially increase rapidly as fluid phase concentration increases (small changes in Ce). The curvature form of the isotherm is known as type I (as is the case with most of the zeolites [25]) or type H of high affinity (called from a mathematical point of view, as it tends to a monolayer [26]). The explication possible for invariable change after of 10 mg·L−1 is due to the less active sites being available at the end of the sorption process and/or the difficulty of the edge molecules (hydrated ion) in penetrating the sorbent. One can, therefore, readily deduce that at low sorbate concentrations, it effectively reduces to a linear isotherm. Alternatively, at high sorbate concentrations it predicts a constant monolayer sorption capacity.
The plot of Ce/qe vs. Ce is presented in Figure 11. The corresponding correlation coefficient and the Langmuir model with its isotherm parameters were calculated and presents in Table 3. The Langmuir model effectively described the sorption data with correlation regression coefficient R equal to 0.999, (which is the measure of goodness-of-fit). The general empirical formula of the Langmuir model is given by

According to the qm values of the Langmuir model, shown in Table 3, the maximum monolayer adsorption

Figure 10. Equilibrium isotherm for lead removal by 3A zeolite.

Figure 11. Langmuir isotherm plot for lead sorbed onto 3A zeolite.

Table 3. Langmuir isotherm parameters for the sorption of Pb2+ onto 3A zeolite.
capacity of zeolite is 14.64 mg·g−1. The Pb (II) experimental uptake was about 14.56 mg·g−1, smaller than the theoretical one, given by Langmuir isotherm model.
The apparent Gibbs free energy of sorption (ΔG°) is the fundamental criterion of spontaneity [24]. Reaction occurs spontaneously at a given temperature if ΔG° for the sorption of Pb2+ by zeolite can be calculated using the following thermodynamic equation:
 (10)
(10)
The value of standard Gibbs free energy change calculated at (21.0˚C ± 0.1˚C) was found to be −4.13 kJ·mol−1. The negative sign for ΔG° is indicative of the spontaneous nature of Pb2+ adsorption on the 3A zeolite.
By plotting logqe vs. logCe, values of Kf and m can be determined from the intercept and slope of the plot. The values of Freundlich parameters obtained applying Equation (6) are presented in Table 4.
The value of m is smaller than 1 and it represents the favorable removal conditions and the higher value for Kf indicates higher affinity for Pb2+ ions. The correlation coefficients obtained from Langmuir and Freundlich indicated that the experimental data fits well to the Langmuir model. Therefore, the coverage of metal ion examined on the surface of the zeolite may be defined as a monolayer. On the other hand, the plot of lnqe against e2 for metal ion sorption on 3A zeolite is shown in Figure 12. The D-R parameters are calculated and listed in Table 5.
The results show than the value of the mean free energy, E, of sorption is 5, which is below the energy of ion exchange reaction. This value indicates the behavior of

Table 4. Freundlich isotherm parameters for the sorption of Pb2+ onto 3A zeolite.

Figure 12. D-R isotherm plot for lead adsorbed onto 3A zeolite at constant temperature.

Table 5. Parameter obtained in the D-R equation.
the lead ion hydrate, which has been retained by psysisorption instead of ionic exchange. We assume that the size of the 3A zeolite and the Pb+2 ion radius make difficult the ion exchange and therefore the value of qm is minor than the found it in the Langmuir isotherm. Moreover this could be because the Langmuir model assumes uniform energies of sorption on the surface and no migration of sorbate in the plane of the surface.
4. Conclusion
The results of the present investigation demonstrate the usability of 3A zeolite as good adsorbent material for removal of Pb (II) from high contaminated water, such as wastewater. The recovery of Pb (II) was >98%, which shows the reliability of the proposed method. Equilibrium isotherms have been determined and tested for different isotherm expressions and the adsorption data were successfully modeled using Langmuir, Freundlich, and Dubinin-Radushkviech (D-R) approaches. Based on the D-R model expression, the maximum adsorption capacity and the mean free energy of the studied ion lead have been determined. The adsorption of ion lead on 3A zeolite is a physic process (Van der Waals interactions) and spontaneous in nature. By considering the fast adsorption-desorption process and the reusability of zeolite, the present work suggests the 3A zeolite, obtained from Venezuelan kaolin, as an alternative material, of relatively low cost for removal of lead ions from wastewater.
5. Acknowledgements
The authors gratefully acknowledge financial support from Instituto de Investigaciones Científicas (IVIC) Caracas-Venezuela Project No 1112, Fondo Nacional de Ciencia, Tecnología e Innovación (FONACIT) CaracasVenezuela Sub-112-223 and No. G-20050000433, to Mr. Miguel Ángel Ramos García of Laboratorio de Difracción y Fluorescencia de Rayos-X (LABDFRX) of Unidad de Caracterización y Estructura de Materiales (UCEM) from Instituto Zuliano de Investigaciones Tecnológicas (INZIT), Ministerio del Poder Popular para el Ambiente (Minamb), Corporación Venezolana de Guayana (CVG) and Mr. José Ramón Díaz Méndez (San José del Palmar).
NOTES