Open Access Library Journal
Vol.06 No.11(2019), Article ID:96662,11 pages
10.4236/oalib.1105909
The Spatial Redistribution of Chemical Elements and Their Isotopes in Layered Intrusions Provided by the Gradients of a Temperature, of a Pressure and of a Deformation, Using Lukkulaisvaara Intrusion (North Karelia) as an Example
Alexander Kh. Zilbershtein1, Alexander A. Chaihorsky2, Vladimir S. Semenov1
1Institute of Precambrian Geology and Geochronology (Russian Academy of Sciences), Saint-Petersburg, Russia
2Nevada Highlands Inc., Reno, NV, USA

Copyright © 2019 by author(s) and Open Access Library Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 8, 2019; Accepted: November 25, 2019; Published: November 28, 2019
ABSTRACT
The spatial local anomalies of the concentration ratios for isotopes 143Nd/144Nd (or εNd) and 87Sr/86S in Lukkulaisvaara intrusion (North Karelia) were discovered. The physical mechanisms and the descriptions of the effect of an arising of those anomalies were developed and presented. The base of the proposed mechanisms is the phenomena of the migration (diffusion) of components, due to the (T, P (or D)) gradients. The description of the processes of an arising of the gradients of a temperature (T), of a pressure (P) and of a deformation (D) in the layered inhomogeneous intrusion during its formation and evolution, were obtained. It was shown that the gradients can induce the spatial redistribution of the chemical elements and their isotopes in the intrusion. The redistribution, in turn, can lead to the observed spatial concentration anomalies for these components. It was first obtained the expressions for the diffusion additions to the isotope’s parameter ε (εNd, in part).
Subject Areas:
Geochemistry
Keywords:
Gradient, Temperature, Pressure, Deformation, Thermal Diffusion, Pressure Diffusion, Division of Isotopes, Lukkulaisvaara

1. Introduction
The spatial anomalies of the concentration of chemical elements and their isotopes in layered intrusions were observed earlier (see, for example [1] [2] [3] ). In this paper we propose a common physical conception for explanation of the arising of such anomalies. The conception is based on the phenomena of the migration (diffusion) of components induced by the gradients of temperature (T) and/or of pressure (P) (or of deformation (D)).
The gradients of temperature (T) and pressure (P) in the layered inhomogeneous intrusion arise from the beginning of its formation up to now. We consider 3 consecutive events, which induce the gradients:
1) The first injection of magma into rocks for the formation of the magma chamber; and injections of additional batches of magma into the formed magma chamber. In this case, the gradients take place near the contact of the magma with the hosting rocks, or (for additional batches of magma) with the formed crystallized intrusion;
2) Consecutive crystallization of different “homogeneous” fields (layers) of the magma in a process of cooling (and decompression) to lithostatic T-P conditions of the hosting rocks. The gradients take place near the boundary between neighbouring different fields (layers);
3) Cooling and the decompression of the crystallized intrusion from the T-P conditions of the crystallization (Tcr, Pcr) to the lithostatic conditions of the hosting rocks (Tl, Pl). Gradients exist and localize near the boundary between the intrusion and the hosting rocks, between the different layers, and between the fields of the different chemical (mineral) compositions (see [1] ).
These gradients can be a reason for the migration of chemical components, by use of the thermal diffusion, the pressure diffusion, the stress-induced diffusion, etc. The migration, in turn, leads to the spatial redistribution of the components and to the arising of the spatial local concentration anomalies of the components.
The concentration anomalies of the platinum group elements in layered intrusions Lukkulaisvaara and Kivakka (North Karelia) were discovered and described (see [1] [3] , for example).
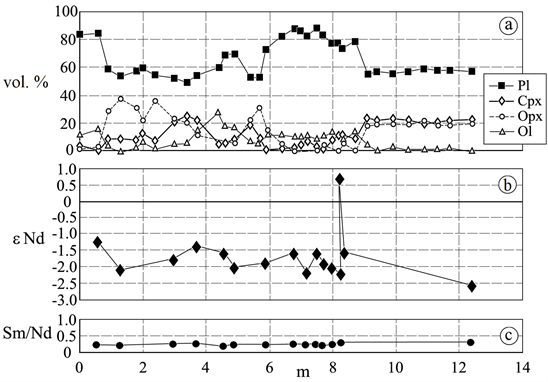
In this paper, we consider as an example of an application of the proposed physical model, the spatial anomalies of concentration ratios for isotopes 143Nd/144Nd (or εNd) and 87Sr/86Sr, which were discovered in Lukkulaisvaara intrusion. Both the anomalies exist in the zone of a contact (near 9 m (see Figure 1)) of the microgabbronorite’s body (as a result of the injection of the additional batch of the magma) with the formed crystallized intrusion (as the hosting rocks). The value of the anomalous ratio for 87Sr/86Sr (is equal to 0.6970 +/− 0.0020) is below the average for the object; the value of the anomalous εNd (see Figure 1) is above the average.
Thus, these anomalies present the enrichment of light isotopes 86Sr and 143Nd in the contact zone.
Figure 1. The observed data of the layered intrusion Lukkulaisvaara (North Karelia): (a) The distribution of the mineral composition along the cut across the lower contact of the microgabbronorite’s body (produced by the additional injection of the magma) with the hosting rocks; (b) The spatial distribution of the εNd; (c) The spatial distribution of the concentration ratio Sm/Nd.
Usually spatial anomalies of various isotopes are explained by an admixture of a xenogeneous substance. For example, in the paper [2] the discovered positive anomaly of the εNd in the Lukkulaisvaara intrusion was discussed, and was explained by the use of the participation of the fluid from the mantle. However, the participation of this fluid cannot explain the localized enrichment of the light isotope 86Sr in this intrusion discovered now.
We propose the alternative physical mechanisms for the description of such effects in closed isotope systems.
2. Mechanisms of the Spatial Redistribution of Chemical Elements and Their Isotopes in Layered Intrusions
The proposed physical conception of the spatial redistribution of components in closed systems supposes the existence of the gradients of a temperature (grad T), and/or of a pressure (grad P) (or of a deformation (grad D)) in the systems. We consider the temporal sequence of the appearance of such gradients in layered intrusions:
1) In the moment of an injection of magma, its temperature and pressure (Тm, Рm) are not equal to these conditions of the hosting rocks (Тl, Рl). It can be supposed, that Тl < Тm. The expression for the (Pm - Pl) was obtained in the form [4] :
(1),
where: r, and rm are the average densities of the hosting rocks (r) and the magma (rm) taking place between the source of the magma and the intrusion; Ро is the pressure of the magma in the source of the magma.
The difference of pressures (Pm ? Pl) = dP > 0 is the uniaxial compression stress (X) for the hosting rocks. The stress Х (= dP) was observed near the boundary of layered intrusions [5] and the boundaries between different layers [1] . By the use of the therm-elastic effect, the stress induces the local change (increase) of the temperature (δT) of the hosting rocks. For constant volume of the system, using the elastic approximation, the expression for the δT presents in the form [6] :
(2)
where: A, and B are the average volumetric thermal expansion coefficient (A), and the average compressibility (B) of the hosting rocks
That is, the injection of the magma induces the change of a pressure dP (see Equation (1)) and/or the change of a temperature δT (see Equation (2)) near the boundary between the magma and the hosting rocks. The changes induce the perpendicular to the boundary gradients of temperature (grad T) and/or of pressure (grad P) in the hosting rocks and in the magma.
In this case, the magma gives its energy to the hosting rocks, i.e. in the liquid magma δT < 0 and the gradient of temperature is positive (grad T > 0).
2) After the formation of the chamber with magma, the magma in the intrusion is cooling and decompressing from (Tm, Pm) to (Tl, Pl). The crystallization of the intrusion (see [7] [8] ) occurs during this process.
The layered intrusion contains different layers and bodies from additional injections of magma. The layers and bodies differ in their chemical (mineral) compositions. The main difference is different ratios of plagioclase’s and pyroxene’s molecules. There are neighboring “plagioclase’s” and “pyroxene’s” layers, etc.
The temperature of crystallization of pyroxenes exceeds the one of plagioclases. Thus, during the cooling and the decompressing the crystallization of the “pyroxene’s” layer occurs before the crystallization of the “plagioclase’s” one. However, a crystallization is accompanied by going out the latent heat of crystallization, that leads to the heating of surroundings. Thus, the crystallization of the “pyroxene’s” layers induces the local increase (ΔT > 0) of the temperature of the liquid magma for “plagioclase’s” layers. The expression for the ΔT was obtained earlier [8] in the form:
(3)
where: Q is the heat of crystallization; Pcr is the pressure of crystallization; dV is the difference of the volume of the melted and crystallized material; Cv is the specific heat at constant volume [9] .
In this case, the gradient of temperature in the liquid “plagioclase’s” magma near the crystallized “pyroxene’s” layer is negative (grad T < 0).
3) Subsequent cooling (to Tl) and decompression (to Pl) of the crystallized layered intrusion leads to the change of the volume. The change is different for different layers, which have the different values of the coefficient of thermal expansion (A) and the compressibility (B). The difference of the change of volume for different layers induces the mutual deformation (D) of neighboring different layers. The expression for the relative mutual deformation (dD) was obtained [1] in the form:
(4)
where: dA and dB are the differences of the values of the A and the B for different neighboring layers.
These deformations are localized near the boundary between different layers or bodies (see [1] ). The zone of the relative tension can be a potential trap for the substance, which can migrate in the intrusion.
Then we consider the influence of such gradients on the spatial distribution of chemical elements and their isotopes in layered intrusions.
The local changes of temperature, of pressure and of deformation (see Equations (1)-(4)) induce the gradients of these parameters. The gradients cause the different kinds of diffusion: a thermal diffusion, a pressure diffusion, a stress-induced diffusion etc. The gradients take place both in the liquid magma and in the neighboring hosting rocks. (We have to note, that the effects of the diffusion induced by the gradients in solid states are very weak, and do not consider in this paper).
We consider the thermal diffusion and the pressure diffusion in magma in the steady state and in the absence of external forces, i.e. when the thermal and/or the pressure diffusion are balanced by the common concentration diffusion.
In this case, for the thermal diffusion, the distribution of the particles of the j-th component (j = 1, 2) in the binary system at established pressure can be found from the following equation [10] :
(5)
where: Kt = Dt/D12 is the thermal diffusion ratio; D12 is the diffusion coefficient; Dt is the thermal diffusion coefficient; Cj = Nj/N is the particle concentration of the j-th component; Nj is the number of particles of the j-th component in unit volume; N is the number of particles of whole mixture (moles of melt) in unit volume.
It is important to note, that if components of the binary system are different isotopes of the same element, then a thermal diffusion can provide the division of the isotopes. The coefficient of isotopes’ division (α) can be expressed in the form [11] :
(6),
where: C1 is the concentration of a light isotope; (1 − C1) is the concentration of a heavy isotope; α (by the definition) is a ratio of the value (C1/(1 − C1)) for the state of the system after the thermal diffusion to this value for the initial state; K is the thermal diffusion constant (K is analogous to the Kt (see Equation (5))); T is the average temperature of the system; dT is the difference of the maximal and the minimal values of the temperature in the system (in particular, the dT can be equal to the δT (Equation (2)) or to the ΔT (Equation (3))).
For a description of concentrations of isotopes in the Earth, the parameter ε is used [12] . This parameter (ε) can be expressed in the form:
,
where: the initial concentration ratio of isotopes is equal to the ratio for the chondrite (CHUR), that supposedly is an equivalent to the ratio for the Earth’s mantle. For example, the ε of Nd (εNd) for a sample is determined and expressed in the form [12] :
By use of the Equation (6) we for the first time obtained the expression for the induced by the thermal diffusion addition (εt) to the ε in the form:
(7)
where: is the coefficient of the isotopes’ division before the thermal diffusion (i.e., when the ε was equal to the average value of the ε in the magma: ).
After the thermal diffusion, the ε can be expressed in the form:
(7а)
From the Equation (7), Equation (7а) one can see, that the local changes (dT) of the temperature (T) of the magma near the boundary with the crystallized material as, for instance, the δT (see Equation (2)) or the ΔT (see Equation (3)), should lead to the local changes of the ε. The sign of the dT determines the sign of the εt. Thus, the injection of the magma produces the negative addition εt for the magma (dT = δT < 0, see above); but in the process of the consequent crystallization of the intrusion, the addition εt for the liquid magma is positive (dT = ΔT > 0, see above).
The light particles (isotopes) migrate to the field of the high temperature and/or the low pressure [11] . For the description of the pressure diffusion we propose to use the analogue of the Equation (5) in the following form (where the change of the sign was taking into account):
(8)
where: Kb = Db/D12 is the pressure diffusion ratio; Db is the pressure diffusion coefficient.
The pressure diffusion could induce the spatial redistribution of isotopes analogically to the thermal diffusion. However, the time of the existence in the liquid magma of the gradient of the pressure (grad P) is significantly less than the time of the existence of the gradient of the temperature (grad T). Thus, for the description of the final observed redistribution of the isotopes we do not take into account the contribution from the pressure diffusion.
3. Discussion
Figure 1 presents the natural observed data of: a) the mineral composition; b) the spatial distribution of the εNd; c) the spatial distribution of the concentration ratio Sm/Nd; ―for the cut across the lower contact of the microgabbronorite’s body (produced by the additional injection of the magma) with the hosting rocks.
The increases of the εNd are observed near the contact of the “more plagioclase’s” fields with the “more pyroxene’s” fields (see the data of Figure 1 for 9 m and 1 m). Thus, the contact zones concentrate the light isotope of Nd (and of Sr too (see above)).
These increases can be explained by use of the thermal diffusion, when the crystallization of the “more pyroxene’s” fields heats the liquid magma of the “more plagioclase’s” fields. Then, the εNd for the contact zones can be obtained and expressed in the form (see Equation (7), Equation (7a)):
(7b)
From the data of Figure 1(b), by use of the Equation (7b), the following values can be easily obtained: ) (the average value of the εNd for the observed interval); εtNd = + 2.4 (for the local maximum of the εNd near 9 m); εtNd = + 0.4 (for the local maximum of the εNd near 1 m). These values and the typical for the Lukkulaisvaara intrusion estimation data of the dT and the T (dT = ΔT > 3.5 × 102 ˚K, T = 1500 ? 1450 ˚K, (see [8] ) allow to estimate the constant of the thermal diffusion (K). The estimation of the K for the given conditions is first obtained, by use of the Equation (7), in the form:
(7c)
Thus, in the present case, the coefficient of the thermal diffusion of the Nd isotopes (Dt) is not more than 1/1000 of the diffusion coefficient of the Nd isotopes (D12) (see Equation (5), Equation (6)).
However, there is an additional possibility to change locally the ε in the closed isotope system of the layered intrusions, i.e., to redistribute the isotopes. This possibility can be provided by the migration of the isotopes which are contained in the fluid to the zones of the low pressure or of the relative tension (i.e., to the traps). The fluid can arise from the cooling and the decompression of the substance of the intrusions. It is well-known, that the migration of the fluid through the porous medium of the intrusions leads to the concentration of the light isotopes in the traps (i.e., in the zones of the relative tension (see Equation (4)). Thus, the migration of the fluid induces the redistribution of isotopes of the fluid.
The physical description of this redistribution of the isotopes, as the local change of the ε, is proposed. We suppose that in the given case the division of the isotopes is induced by the diffusion in the field of the changeable deformation (D). By use of the description of the pressure diffusion (Equation (8)), the coefficient of the isotopes’ division for the fluid (αbf) can be first presented in the form:
(9)
where: Kbf is the pressure diffusion ratio for components of the fluid; dP = −MdD (M is the bulk modulus of the material of the field of the tension); dD is determined by use of the Equation (4); P is the pressure of the process.
The Equation (9) allows to obtained for the first time the expression for the addition (εbf) to the value of the ε(εf1), which was in the fluid before the described diffusion process: , in the form:
(10)
where: .
For the zones of the tension of the intrusions, where dD > 0, the addition εbf is positive (εbf > 0) and the final value of the ε in the fluid (equal to (εf1 + εbf)) increases (see Equation (10)). The zones of the tension in layered intrusions arise by the cooling and the decompression of the intrusions. They take place near the boundaries between the different layers and/or between the different fields of different chemical or mineral compositions (see [1] ). In the given case for the Lukkulaisvaara intrusion these fields demonstrate different concentration ratios of the pyroxene’s and the plagioclase’s molecules. The boundaries take place near 1 m and near 9 m (see Figure 1(a)). Thus, the possible change of the ε from the fluid can be near these places (near 1 m and near 9 m (see Figure 1(a), and Figure 1(b)). The value of the ε1 is equal to the value of the εf1 (ε1 = εf1) in the considered closed isotope system. Thus, the εbf can be the positive addition to the εt (see Equation (7), Equation (7b)) near the boundaries placed near 1 m and 9 m (see Figure 1).
It is important that the fluid can migrate a long time: from the birth of the fluid up to now, and the time defines the value of the diffusion displacement.
4. Conclusions
The common physical conception and diffusion mechanisms are proposed for the description of the spatial redistribution of the chemical elements and their isotopes in the inhomogeneous layered intrusions. The base of the mechanisms is the diffusion of the components due to the gradients of temperature, pressure, and deformation. Such gradients were described in the layered intrusions. The equations were obtained for the description of the thermal diffusion and first of the pressure diffusion and of the deformation diffusion of the chemical elements and their isotopes (Equations (5)-(9)). These equations allowed first to describe the arising of the spatial local concentration anomalies of the isotopes (Equation (7), Equation (10)). Such anomalies for 143Nd/144Nd and 87Sr/86Sr in the layered intrusion Lukkulaisvaara (North Karelia) were observed and described. For the first time, the evident expressions for the thermal diffusion addition (εt) (Equation (7)) and for the deformation diffusion addition (εbf) (Equation (10)) to the ε (εNd, in part) were obtained. The thermal diffusion constant (K) for isotopes of Nd in the intrusion Lukkalaisvaara was first estimated (Equation (7c)).
We have to note that the proposed diffusion mechanisms and the physical descriptions of the arising of the spatial concentration anomalies of chemical elements and their isotopes do not suppose the introduction of the xenogeneous substance into the system (the layered intrusion). Thus, in part, the proposed approach is permitted for the first time to explain the arising of the observed localized spatial isotopes’ anomalies of 143Nd/144Nd and of 87Sr/86Sr in Lukkulaisvaara intrusion by the spatial redistribution of the components without a participation of the xenogeneous substance, i.e. in the closed system of the isotopes.
The described effects have to be taken into account for the solution of various geological problems. In part, the described diffusion processes can lead to local spatial concentration anomalies for isotopes of Nd and of Sr in various inhomogeneous geological objects that should be taken into account by use of the “Sm/Nd” and the “Rb/Sr” geochronological methods.
It is very interesting to find out the T-P-X conditions of those diffusion processes for the isotopes of the other elements (U, Pb, etc.) in the crystals, which are used for the geochronology. In part, it is important to study the diffusion (and the division) of isotopes of Pb (and/or of U) in zircon, where not only the described above thermal diffusion and the pressure diffusion are possible, but the stress-induced diffusion (that can provide the enrichment (for X > 0) of the heavy isotopes of the volume of the crystal) is possible too.
Those studies and the present paper can, in part, permit to find the Earth’s objects for the more correct isotope geochronology.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Zilbershtein, A.Kh., Chaihorsky, A.A. andSemenov, V.S. (2019) The Spatial Redistribution of Chemical Elements and Their Isotopes in Layered Intrusions Provided by the Gradients of a Temperature, of a Pressure and of a Deformation, Using Lukkulaisvaara Intrusion (North Karelia) as an Example. Open Access Library Journal, 6: e5909. https://doi.org/10.4236/oalib.1105909
References
- 1. Zilbershtein, A.Kh., Glebovitsky, V.A., Semenov, V.S. and Velikoslavinsky, S.D. (1999) Deformation Non Uniformity during Cooling and Decompression of Magmatic Intrusions and the Influence on the Fluid Distribution. Doklady Earth Sciences, 366, 506-509.
- 2. Epifanova, T.A., Kazanov, O.V. and Savatenkov, V.M. (2007) Sources of a Matter of Ore-Bearing Rocks of Layered Massive Lukkulaisvaara: Results of Sm-Nd Isotopy. Materials of the IVIII Conference “Actual Problems of Precambrian Geology, Geophysics and Ecology”. Saint-Petersburg, 222-225.
- 3. Semenov, S.V., Glebovitsky, V.A., Kol’tsov, A.B., Semenov, V.S., Korneev, S.I. and Savatenkov, V.M. (2008) Metasomatic Processes in the Lukkulaisvaara Layered Intru-sion, Russia, and Formation of Low-Sulfide PGE Mineralization. Geology of Ore Deposits, 50, 283-310. https://doi.org/10.1134/S1075701508040016
- 4. Zilbershtein, A.Kh., Semenov, V.S., Glebovitsky, V.A. and Dech, V.N. (2014) Injection of Additional Batches of Magma into Formed Magma Chamber: The Case Study of Lukkulaisvaara Intrusion, North Karelia. Physics of the Solid Earth, 50, 305-309. https://doi.org/10.1134/S1069351314010108
- 5. Berkovsky, A.N., Zilbershtein, A.Kh., Glebovitsky, V.A., Semenov, V.S. and Shalaev, V.V. (1999) Assessment of Magma Pressure during Intrusion: A Case Study of the Kivakka, Lukkulaisvaara and Tsipringa Plutons, Northern Karelia. Doklady Earth Sciences, 367, 601-604.
- 6. Nye, J. (1987) Physical Properties of Crystals. Clarendon Press, Oxford, 329.
- 7. Choban, E.A., Semenov, V.S. and Glebovitsky, V.A. (2006) Rhythmical Exfoliation in Magmatic Chamber of Basite-Hiperbasite’s Intrusions by the Use of Diffusion and Resurveyed Convection. Physics of the Earth, 5, 9-24.
- 8. Zilbershtein, A.Kh., Semenov, V.S., Glebovitsky, V.A., Dech, V.N. and Semenov, S.V. (2009) Temperature in Magmatic Chamber in the Time of Magma’s Crystallization. Bulletin of the University of St. Petersburg, 7, 3-14.
- 9. Robie, R.A. and Hemingway, B.S. (1995) Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar Pressure and at Higher Temperatures. U.S. Geol. Survey, Washington DC, Bulletin 2131.
- 10. Grew, K.E. and Ibbs, T.L. (1952) Thermal Diffusion in Gases. Cambridge University Press, Cambridge, 143 .
- 11. Chemla, M. and Perie, J. (1976) La separation des isotopes. Press Universitaires de France, 168.
- 12. Wasserburg, D.J. and De Paolo, G.J. (1976) Nd isotopic Variations and Petrogenetic Models. Geophysical Research Letters, 3, 249-252.
https://doi.org/10.1029/GL003i005p00249 - 13. Faak, K., Coogan, L. and Chakraborty, S. (2015) Near Conductive Cooling Rates in the Upper-Plutonic Section of Crust Formed at the East Pacific Rise. Earth and Planetary Science Letters, 423, 36-47. https://doi.org/10.1016/j.epsl.2015.04.025
- 14. Samperton, K., Schoene, B., Cottle, J., Keller, C.B., Crowley, J. and Schmitz, M. (2015) Magma Emplacement, Differentiation and Cooling in Middle Crust: Integrated Zircon Geochronological-Geochemical Constraints from the Bergell Intrusion, Central Alps. Chemical Geology, 417, 322-340.
https://doi.org/10.1016/j.chemgeo.2015.10.024 - 15. Bushuev, A.N., Zhukovin, Z.V. and Elkin, O.V. (2015) Determining the Diffusion Coefficient of Praseodymium Ions in Equimolar NaCl-KCl-PrCl3 Melt. Transacrtions of Kol’sky Centre of Russ. Acad. Sci., 31, 212-213.
Appendix
For the additional confirmation of the correctness of the proposed mechanisms and descriptions, we provide the rough estimation of the diffusion displacement for the described natural conditions.
In according to data of the papers [13] [14] the velocity (vc) of cooling of the intrusions is about 10−11 ˚K/s. Using the linear approximation of the cooling one can obtain the estimation of duration (time) of the different described diffusion processes of isotopes division.
In part the estimation of the duration (t) of the thermal diffusion during the consecutive crystallization of different layers (“pyroxene’s” and “plagioclase’s”) is determined by the difference (dTcr) of the temperature of their crystallization (about 102 ˚K): t = dTcr/vc = 1013 s.
In this case the diffusion coefficient of Nd in the magma (D12) is approximately equal to the well known diffusion coefficient of ions of Pr (resembling to Nd) in the melt (NaCl ? KCl ? PrCl3) at 1100 - 1200 ˚K (T) and 1 bar (P): (2.2 +/− 0.5). 10−9 m2/s (see [15] ).
The value of the diffusion displacement (L) was roughly estimated by the use of: D12 = 10−9 m2/s; K = 10−3 (see Equation (7c)); t = 1013 s (see above). Using these data, the estimation of the L can be obtained from the following expression: , and is equal to 101/2 m, that is in a good accordance to the described above data (see (Figure 1(b))).
We are very grateful to V. Chernin and Ass. Prof. N. Korovina for the help in preparation of this paper.