Advances in Microbiology
Vol.06 No.14(2016), Article ID:73015,16 pages
10.4236/aim.2016.614100
Synergistic Effect of Combined Antibiotics against Some Selected Multidrug Resistant Human Pathogenic Bacteria Isolated from Poultry Droppings in Akure, Nigeria
Funmilola Oluyemi Omoya, Kehinde Oluyemi Ajayi
Department of Microbiology, Federal University of Technology, Akure, Nigeria

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 16, 2016; Accepted: December 24, 2016; Published: December 27, 2016
ABSTRACT
Antibiotic resistant bacteria pass between humans, between animals and between humans and animals in both directions, the use of antibiotics in poultry has contributed to multiple antibiotic resistant in pathogenic bacteria and use of two antibiotics might prevent the emergence of resistance to either. In this study, synergistic effect of combined antibiotics against multidrug resistant human pathogenic bacterial isolates from poultry droppings in Akure, Nigeria was examined. Collection of samples, isolation and identification of bacteria were carried out using standard microbiological method, antibiotic sensitivity test was performed by disc diffusion method and zone of inhibition was used to interpret the sensitivity test as resistant, susceptible or intermediate while combined effects of two antibiotics were investigated by macrobroth dilution and checkerboard assay methods while the synergetic effects of combined antibiotics were calculated using Fractional Inhibitory Concentration (FIC) and percentage synergistic interaction was calculated. All the ten (10) species of bacterial isolates were multidrug resistant and are less resistant to ofloxacin. The highest percentage synergistic interactions observed were Ofloxacin + Amoxicillin (90%), Ciprofloxacin + Amoxicillin (90%), Tetracycline + Amoxicillin (70%), Tetracycline + Augmentin (80%), Cotrimoxazol + Amoxicillin (50%), Cotrimoxazol + Augmentin (70%), Chloramphenicol + Amoxicillin (70%) and Chloramphenicol + Augmentin (80%). Poultry droppings is a potential source of human pathogenic bacteria, high frequency of multiple antibiotic resistance bacteria observed in this study is of great treat to man as this may cause the treatment of infection caused by these bacteria to be difficult. Combination of beta-lactam antibiotic with fluoroqunolones, tetracycline, Chloramphenicol and Cotrimoxazole was synergetic and this will reduce dose related toxicity and prevent resistance to single antibiotic.
Keywords:
Antibiotic Resistant, Pathogenic Bacteria, Multidrug Resistant, Synergistic Effect, Combined Antibiotic

1. Introduction
In reality, increasing resistance levels are driven by antibiotic use in all sectors: in humans, in community and in hospitals, on farms and in companion animals. Although resistance in human infections is mainly caused by human antibiotic use, for a range of bacteria, antibiotic use in farm-animal contributes significantly and for some infections, antibiotic use in farm-animal is the main source of resistance. Antibiotic-resistant bacteria pass between humans, between animals and between humans and animals in both directions mostly through the food chain much more frequently than once realized. Copies of antibiotic-resistance genes can also move between bacteria, and this exchange can occur in the human gut, so in some cases the bacteria causing a human infection will not be of farm-animal origin, but the resistance will be [1] [2] [3] .
The use of antimicrobial agents in veterinary practice improves poultry health and production [4] [5] . The development of resistance to antibiotics in bacteria led to a discussion about the careful use of antimicrobial agents, especially in veterinary medicine, nutrition and agriculture [6] . It is very important to monitor the resistance to antibiotics not only in human bacterial pathogens but also in pathogenic and commensal bacteria of animal origin [7] . The rapid emergence of resistance to antibiotics amongst pathogens generates visions of the potential post-antibiotic era threatening present and future medical advances [8] .
Since there is a growing awareness of public health concerns associated with the emergence of drug-resistant strains of bacteria [8] and antibiotic resistance is currently involving most of the known classes of natural and synthetic antibiotics [9] , there is an urgent need for antibiotics with novel scaffolds and mechanism of actions. In response to identifying alternative means to combat these situations, combination therapy or polypharmacy has been acknowledged as a promising therapeutic approach [10] [11] . Combining two antibiotics may result in synergism, indifference or antagonism. In the case of synergism, microbial inhibition is achieved at concentrations below that for each agent alone [11] , hence synergy is defined as significantly greater activity provided by two agents combined than that provided by the sum of each agent alone [12] [13] .
The clinical use of combination of antibiotic therapy for bacterial infections in general can be divided into two categories [13] . In the first category, such therapy is used to improve clinical outcomes of infections with strains that are susceptible to one or more individual antibiotics. The primary rationale for combining two agents is to enhance the activity or either by achievement of a synergistic effect. A secondary rationale is to allow lower doses of either antibiotic to reduce toxicity. Third, use of two antibiotics
might prevent the emergence of resistance to either. The second category of antibiotic combination use has evolved during the last decade, during which certain clinical species have become resistant to all available antibacterial agents or to all except a single agent, combination therapy is occasionally recommended to prevent resistance emerging during treatment [11] [13] [14] .
To avoid unpleasant pharmacological and therapeutic actions related to drug-drug interactions [15] and forestall therapeutic failures, development of bacterial resistance and increased cost of treatment often associated with the inept polypharmaceutical practices [11] , and researches in synergistic mechanisms of action for controlling microbial infections becomes indispensable. Tetracycline, Chloramphenicol, Amoxicillin and Cotrimoxazole are widely used to treat community and healthcare-associated infections. The emergence and dissemination of antimicrobial resistance to these antibiotics represents a significant threat to human health [16] . This study therefore investigate the synergetic effect of combinations of different antibiotics on some selected multiple antibiotic resistance bacterial isolates from poultry droppings in Akure, Nigeria.
2. Materials and Methods
2.1. Sample Locations
Akure is the largest city and capital of Ondo State, located in south-west Nigeria. Akure lies about 70˚15 north of the equator and 50˚15 east Meridian. The city has a population of 588,000 which is 0.305% of Nigeria population based on 2006 population census, the people are of Yoruba ethnic group and are situated in the tropic rainforest. The city is a trade center for farmers where cocoa, bananas, palm oil, yams, cassava, corn, cotton and tobacco are mostly cultivated, the residents also engaged in various economic activities such as trading, transportation business, civil service and education. During this research, Six hundred and eighty four (684) fresh poultry droppings were collected from forty eight (48) different poultries across nine (9) different locations, namely; FUTA, Aba, Apatapiti, Ijoka, Oritaobele, Ado road, Ondo road, Alagbaka and Lafe where poultry farming are mostly located in Akure metropolis.
2.2. Sample Collection
Fresh droppings of poultry birds were collected from poultries as described by [17] , [18] , labeled appropriately with the source, time and date of collection. They were transported to microbiology research laboratory of Federal University of Technology, Akure (FUTA) within one hour of collection for microbiological analyses.
2.3. Isolation and Identification of Bacteria from Poultry Droppings
Sample collected in McCartney bottle was gently shake and stirred with sterile glass rod until the droppings mixed thoroughly, aliquot (1.0 ml) was transferred into the test tube containing 9.0 ml of sterile distilled water and diluted serially in one-tenth stepwise to 10−7 dilution factor and 1.0 ml each of dilution 10−5 , 10−6 and 10−7 was pure plated on Nutrient agar and some selective and differential media (Salmonella Shigella agar, Eosin Methylene Blue agar, MacConkey agar and Manitol Salt ager), the plates were inverted and incubated aerobically at 37˚C for 24 hours after which the plates were examined for growth. The bacteria colonies that developed on all media plates were subculture by streaking on a freshly prepared nutrient agar plates until pure colonies were obtained, different biochemical test were carried out for further identification according to procedure highlighted by [19] .
2.4. Antibiotic Susceptibility Test
Antibiotics susceptibility test of all the isolates was determined by the disk diffusion method and interpreted as susceptible, intermediate and resistant as described by Clinical Laboratory Standardization Institute [20] . Gram negative pathogens were tested against the following antibiotics; Tetracycline (30 μg), Ofloxacin (30 μg), Gentamicin (20 μg), Chloramphenicol (30 μg), Augmentin (30 μg), Ceftriazone (30 μg), Nitrofuratoin (300 μg), Cotrimoxazole (25 μg), Ciprofloxacin (10 μg) and Amoxicillin (30 μg) while gram positive isolate were tested against Cotrimoxazole (25 μg), Erythromycin (10 μg), Gentamicin (20 μg), Augmentin (30 μg),Streptomycin (10 μg), Cloxacilin (5 μg) Tetracycline (30 μg) and Chloramphenicol (30 μg). Multiple antibiotics resistance was described in this study as resistance to three of more classes of antibiotics.
2.5. Antibiotics Used in This Research for Synergistic Assay
Commercially available antibiotics were gotten from a standard and registered pharmaceutical store located at FUTA south gate, Akure. Tetracycline, Chloramphenicol, Ofloxacin, Ciprofloxacin, Amoxicillin, Augmentin and Cotrimoxazole were used. Stock antibiotic solutions were prepared and dilutions made as described by [20] .
2.6. Determination of Minimum Inhibitory Concentration (MIC)
The MIC of different antibiotics were determined against the isolates by a standard broth dilution method, briefly serial two fold dilution of the antimicrobial agents was prepared in Mueller Hinton broth (MHB) in test tubes. Culture were grown over night at 37˚C, the standard inocula were prepared by direct suspension in MHB and adjusted with sterile saline until the turbidity matched a 0.5 MacFarland standard. Drug-con- taining and control test tubes were inoculated with the diluted bacteria suspension that gave a final concentration of ~105 CFU/ml. The MIC was determined as the lowest concentration of each drug at which no visible growth was observed by visual examination of the plats after 24 hours incubation at 37˚C, the turbidity were also read spectrophotometrically at 540 nm and optical density (OD) values were recorded to further buttress the physical examination of the broth turbidity. The lowest antibiotic concentration in which there was no visible growth or lowest absorbance was recorded as MIC in mg/ml [11] [20] [21] .
2.7. MIC of Combined Antibiotics on Multidrug Resistant Bacteria
Checkerboard assay was employed using tube dilution [22] . The range of drug concentration used in the checkerboard assay was such that the dilution range encompassed the MIC for each drug used in the analysis. Representative pairs of antibiotics known to show either synergism or antagonism when acting on certain bacteria in broth were tested for their combined effect on bacteria [11] . The MIC of the combined antibiotics was determined as described by [23] .
2.8. Calculation of Fractional Inhibitory Concentration Index (FIC)
The FIC of combined antibiotics was determined by a standard checkerboard method The FIC calculated according to the equation [24] :
FICindex = MIC of antibiotic in combination/MIC of antibiotic alone.
FIC of the two drugs = FIC index of drug A + FIC index of drug B.
Synergism is defined as FIC ≤ 0.5, additive as FIC > 0.5 and ≤ 1, indifference as FIC > 1 and ≤ 2 and antagonism is defined as FIC > 2 [21] .
2.9. Calculation of Percentage Synergetic Interaction
In this study, two antibiotics were combined in these four different ways: (Tetracycline + Chloramphenicol, Tetracycline + Ofloxacin, Tetracycline + Ciprofloxacin, Tetracycline + Amoxicillin, Tetracycline + Augmentin and Tetracycline + Cotrimoxazole), (Chloramphenicol + Tetracycline, Chloramphenicol + Ofloxacin, Chloramphenicol + Ciprofloxacin, Chloramphenicol + Amoxicillin, Chloramphenicol + Augmentin and Chloramphenicol + Cotrimoxazole), (Amoxicillin + Chloramphenicol, Amoxicillin + Ofloxacin, Amoxicillin + Ciprofloxacin, Amoxicillin + Tetracycline, Amoxicillin + Augmentin and Amoxicillin + Cotrimoxazole) and (Cotrimoxazole + Chloramphenicol, Cotrimoxazole + Ofloxacin, Cotrimoxazole + Ciprofloxacin, Cotrimoxazole + Amoxicillin, Cotrimoxazole + Augmentin and Cotrimoxazole + Tetracycline) for antibiotic synergetic interactions against multi drug resistant bacterial isolates.
Percentage (%) synergetic interaction = a/b × 100
where “a” = number of synergetic interaction against the tested bacterial isolates,
“b” = total number of tested bacterial isolates.
3. Results
3.1. Bacteria Isolated form Poultry Droppings
During this research, samples of poultry droppings was collected from layers, broilers and free range chicken in different locations, all within Akure metropolis. One hundred and fifty-seven (157) bacteria were isolated and identified as: Escherichia coli, Kebsiella spp., Proteus spp., Enterobacter spp., Salmonella spp., Shigella spp., Citrobacter spp., Pseudomonas sp., Seratia sp., Staphylococcus spp., Micrococcus luteus and Bacillus spp. All the bacterial isolates were multidrug resistant, however, Table 1 revealed ten selected bacterial isolates that were multidrug resistant; however, they are susceptible to ofloxacin compared to other antibiotics except Escherichia coli and Pseudomonas aeruginosa which were also resistant to all the tested antibiotics.
Table 1. Antibiotic susceptibility pattern of selected multidrug resistant bacterial isolates
Key: S = Susceptible; I= Intermediate; R= Resistant; - = Not determined.
3.2. Minimum Inhibitory Concentration of Selected Multiple Antibiotic Resistant Bacteria
Minimum Inhibitory Concentration was carried out on selected multiple antibiotic resistant bacteria isolated from poultry droppings and presented in Table 2. The range of MIC observed in Augmentin is 64 µg/ml to 128 µg/ml, the lowest was observed in Escherichia coli and Staphylococcus aureus while others are 128 µg/ml. The MIC observed in bacteria against ofloxacin and ciprofloxacin were generally lower than what was observed in other antibiotics, the range of MIC is from 1 µg/ml to 16 µg/ml. However, the MIC of Pseudomonas aeruginosa was generally higher than other isolates against all antibiotics used.
3.3. Synergistic Interaction of Amoxicillin in Combination with Other Antibiotics against Selected Multiple Antibiotic Resistant Bacteria
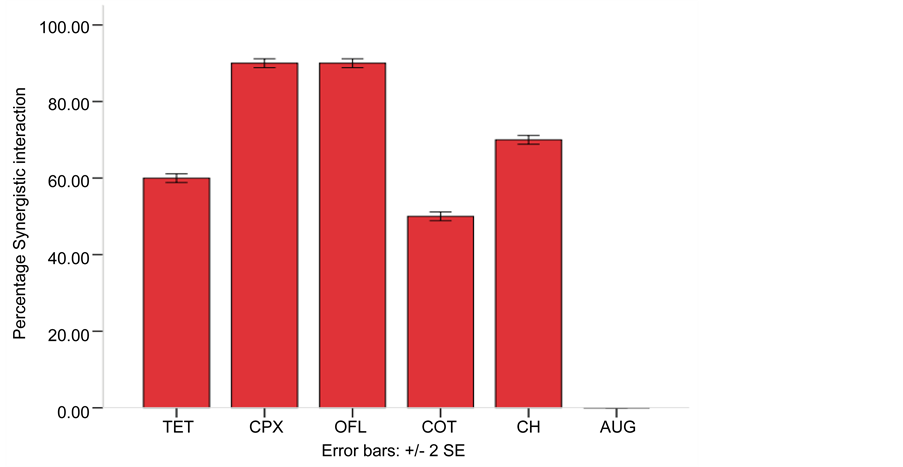
Amoxicillin a beta-lactam antibiotic was combined with other commonly used antibiotics (Augmentin + Amoxicillin, Ofloxacin + Amoxicillin, Ciprofloxacin + Amoxicillin, Tetracycline + Amoxicillin, Cotrimoxazole + Amoxicillin and Chloramphenicol + Amoxicillin) and tested against representative isolates of multidrug resistant bacteria for synergistic interaction. The result in Table 3 revealed the FIC of the combined antibiotics while Figure 1 showed that the combined effect of amoxicillin with other antibiotics is synergistic except augmentin, however, the highest percentage synergistic in-
Table 2. Minimum inhibitory concentration (MIC) of selected multiple antibiotic resistant bacteria isolated from poultry droppings
Table 3. Fractional inhibitory concentration (FIC) of amoxicillin in combination with Tetracycline, Ciprofloxacin, Ofloxacin, Cotrimoxazole, Chloramphenicol and Augmentin against selected multiple antibiotic resistant bacteria.
Key: AUG―Augmentin, OFL―Ofloxacin, CPX―Ciprofloxacin, TET―Tetracycline, COT―Cotrimoxazole, CH―Chloramphenicol.
Figure 1. Synergistic interaction of Amoxicillin in combination with Tetracycline, Ciprofloxacin, Ofloxacin, Cotrimoxazole, Chloramphenicol and Augmentin against selected multiple antibiotic resistant bacteria. Key: AUG―Augmentin, OFL―Ofloxacin, CPX―Ciprofloxacin, TET―Tetra- cycline, COT―Cotrimoxazole, CH―Chloramphenicol.
teraction is between the combination of Ofloxacin + Amoxicillin (90%) and Ciprofloxacin + Amoxicillin (90%).
3.4. Synergistic Interaction of Tetracycline in Combination with Other Antibiotics against Selected Multiple Antibiotic Resistant Bacteria
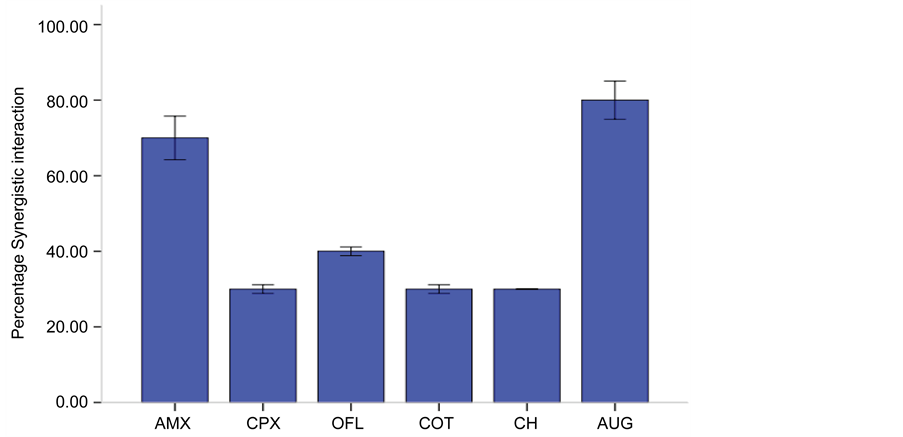
Tetracycline was used in combination with other antibiotics for synergistic interaction, the result of FIC is presented in Table 4 and Figure 2 showed percentage synergistic interaction. All the combinations showed a synergistic interaction but there were high percentage synergistic interaction between Tetracycline + Amoxicillin (70%) and Tetracycline + Augmentin (80%).
3.5. Synergistic Interaction of Cotrimoxazol in Combination with Other Antibiotics against Selected Multiple Antibiotic Resistant Bacteria
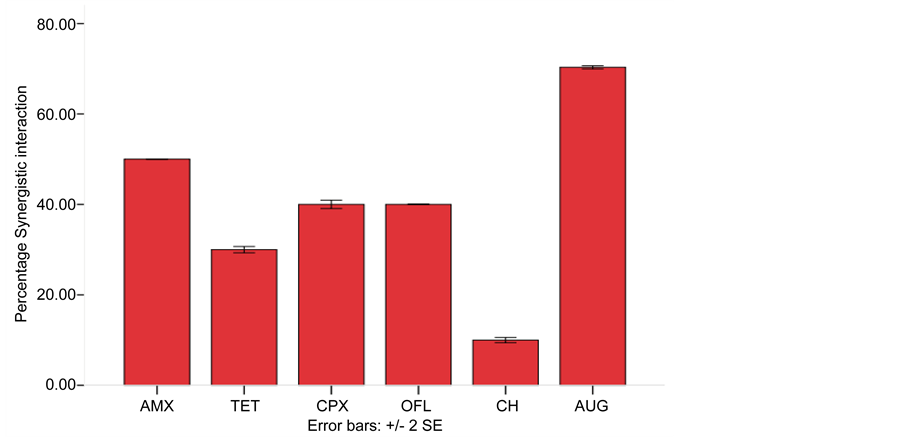
Cotrimoxazol was used in combination with other antibiotics for synergistic interaction, the result of FIC is presented in Table 5 and Figure 3 showed percentage synergistic interaction. All the combinations showed a synergistic interaction but there were high percentage synergistic interaction between combination of Cotrimoxazol + Amoxicillin (50%) and Cotrimoxazol + Augmentin (70%).
3.6. Synergistic Interaction of Chloramphenicol in Combination with Other Antibiotics against Selected Multiple Antibiotic Resistant Bacteria
Chloramphenicol was used in combination with other antibiotics for synergistic inte-
Table 4. Fractional inhibitory concentration (FIC) of Tetracycline in combination with Amoxicillin, Ciprofloxacin, Ofloxacin, Cotrimoxazole, Chloramphenicol and Augmentin against selected multiple antibiotic resistant bacteria.
Key: AUG―Augmentin, OFL―Ofloxacin, CPX―Ciprofloxacin, TET―Tetracycline, COT―Cotrimoxazole, CH―Chloramphenicol.
Figure 2. Synergistic interaction of Tetracyclinein combination with Amoxicillin, Ciprofloxacin, Ofloxacin, Cotrimoxazole, Chloramphenicol and Augmentin against multiple antibiotic resistant bacteria. Key: AUG―Augmentin, OFL―Ofloxacin, AMX―Amoxicillin, CPX―Ciprofloxacin, AMX―Amoxicillin, COT―Cotrimoxazole, CH―Chloramphenicol.
Table 5. Fractional Inhibitory Concentration (FIC) of Cotrimoxazole in combination with Amoxicillin, Ciprofloxacin, Ofloxacin, Tetracycline, Chloramphenicol and Augmentin against selected multiple antibiotic resistant bacteria.
Key: AUG―Augmentin, OFL―Ofloxacin, CPX―Ciprofloxacin, TET―Tetracycline, COT―Cotrimoxazole, CH―Chloramphenicol.
Figure 3. Synergistic interaction of Cotrimoxazol in combination with Amoxicillin, Ciprofloxacin, Ofloxacin, Tetracycline, Chloramphenicol and Augmentin against Multiple Antibiotic Resistant Bacteria. Key: AUG―Augmentin, OFL―Ofloxacin, AMX―Amoxicillin, CPX―Ciprof- loxacin, AMX―Amoxicillin, TET―Tetracycline, CH―Chloramphenicol.
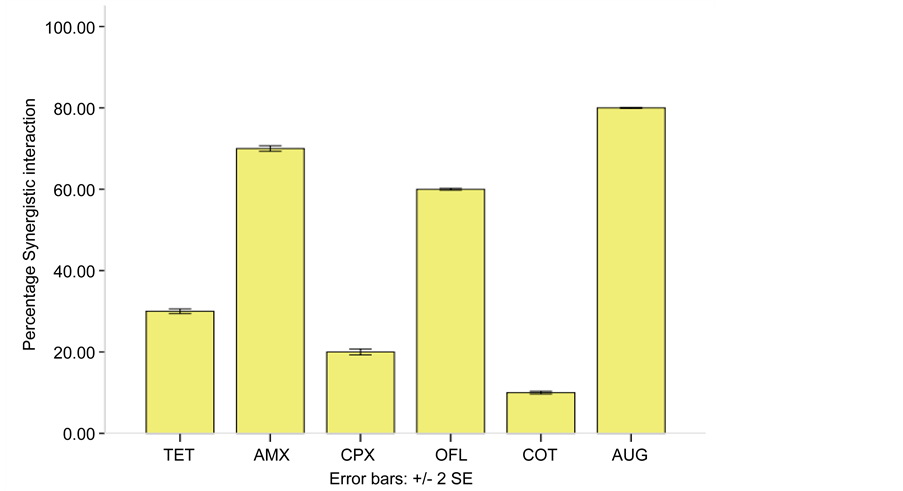
raction, the result of FIC is presented in Table 6 and Figure 4 showed percentage synergistic interaction. All the combinations showed a synergistic interaction but there were high percentage synergistic interaction between combination of Chloramphenicol + Amoxicillin (70%) and Chloramphenicol + Augmentin (80%).
4. Discussions
The result of this work revealed that several pathogenic bacteria were present in poultry droppings, isolated bacteria were also in accordance with the findings of [7] [17] [18] [25] [26] . The presence of these bacteria in poultry droppings could be due to the contamination of the feed and water used in feeding the poultry fowl, contamination may also be caused by the body micro floral of poultry attendants. Poultry meat can be contaminated by droppings with a variety of foodborne pathogens that may cause human illness following ingestion and is due to handling of raw meat, undercooking or mishandling of the cooked product [17] [27] .
Table 6. Fractional inhibitory concentration (FIC) of Chloramphenicol in combination with Amoxicillin, Ciprofloxacin, Ofloxacin, Tetracycline, Cotrimoxazole and Augmentin against selected multiple antibiotic resistant bacteria.
Key: AUG―Augmentin, OFL―Ofloxacin, CPX―Ciprofloxacin, TET―Tetracycline, COT―Cotrimoxazole, CH―Chloramphenicol.
Figure 4. Synergistic Interaction of Chloramphenicol in Combination with Amoxicillin, Ciprofloxacin, Ofloxacin,Tetracycline, Cotrimoxazol and Augmentin against Multiple Antibiotic Resistant Bacteria. Key: AUG―Augmentin, OFL―Ofloxacin, AMX―Amoxicillin, CPX―Ciproflo- xacin, AMX―Amoxicillin, TET―Tetracycline, COT―Cotrimoxazol.
In this study, bacterial isolates showed a very high resistant to augmentin, ceftriaxone, nitrofuratoin, gentamicin, amoxicillin, tetracycline, cotrimoxazole, ciprofloxacin and chloramphenicol while frequency of resistance in ofloxacin is low. Multiple antibiotic resistances observed in this work are in agreement with [28] [29] [30] . Therefore it can be suggested that antibiotic resistance observed in this study is as a result of continual use of antibiotics in poultry. The development of multiple antibiotic resistance in these pathogenic bacteria of poultry origin (e.g. E. coli, Pseudomonas aeruginosa, Klebsiella spp. Proteus spp., Salmonella spp., Shigella spp. and Staphylococcus spp.) constitutes a public health risk, as it may potentially affect the efficacy of drug treatment in humans [31] , high frequency of antibiotic resistance in these pathogenic bacteria may contribute to epidemiology of infections caused by these bacteria in Akure, Nigeria.
Furthermore, as a remedy to the multiple antibiotic resistance observed in this study to various antibiotics especially amoxicillin, tetracycline, cotrimoxazol and chloramphenicol because they are the most used antibiotics in developing countries and are relatively cheap, the emergence and dissemination of antimicrobial resistance to these antibiotics represents a significant threat to human health [16] . Combination effect of amoxicillin, tetracycline, cotrimoxazol and chloramphenicol with other antibiotics were carried out, against the representative of multiple antibiotic isolates and synergistic interaction were observed. This is in agreement with other findings [11] [13] [21] .
Amoxicillin a beta-lactam antibiotic was combined with other commonly used antibiotics (Augmentin + Amoxicillin, Ofloxacin + Amoxicillin, Ciprofloxacin + Amoxicillin, Tetracycline + Amoxicillin, Cotrimoxazole + Amoxicillin and Chloramphenicol + Amoxicillin) showed that the combined effect of amoxicillin with other antibiotics is synergistic except augmentin, however, the synergistic interaction between combination of Ofloxacin + Amoxicillin and Ciprofloxacin + Amoxicillin are 90%. Amoxicillin is β-lactam that impairs the development of bacterial cell wall by interfering with transpeptidase enzyme responsible for the formation of the cross-links between peptidoglycan strands [21] . Several studies have demonstrated synergistic interaction between β-lactams and other antibacterial agents, in which the inhibition of cell wall synthesis by β-lactams significantly enhanced the uptake of other drugs [21] . Similar phenomenon might happen in this study in which synergism of the combinations might arise as a result of increased cellular uptake of ofloxacin and ciprofloxacin due to Amoxicillin induced inhibition of bacterial cell wall synthesis. This will reduce the doses and the risk of dose-related toxicity of fluoroqunolones and enhanced efficiency [32] .
Tetracycline a bacteriostatic antibiotic that cause inhibition of protein synthesis by binding to the 30S ribosomal subunit was combined with other antibiotics (Augmentin + Tetracycline, Ofloxacin + Tetracycline, Ciprofloxacin + Tetracycline, Amoxicillin+ Tetracycline, Cotrimoxazole + Tetracycline and Chloramphenicol + Tetracycline), the result revealed a synergistic interactions with all combinations but the highest synergistic interactions was observed in Tetracycline + Amoxicillin (70%) and Tetracycline + Augmentin (80%). This might be as a result of increase permeability caused by amoxicillin and augment in allowing tetracycline to penetrate and inhibit protein synthesis. This was also reported by [11] [33] , the ability of these combinations to inhibit Gram- negative and Gram-positive as well as those considered resistant to amoxicillin and augmentin to a great extent showed that combined therapy of tetracycline + amoxicillin and tetracycline + augmentin against infectious agents could be more effective than their monotherapy as well as killing pathogens before resistance development.
Also, Cotrimoxazol a bacteriostatic antibiotic that competitively inhibit dihydropteroate synthetase (DHPS), which is necessary for the synthesis of the required nutrient (folate) in bacteria was combined with other antibiotics (Augmentin + Cotrimoxazol, Ofloxacin + Cotrimoxazol, Ciprofloxacin + Cotrimoxazol, Amoxicillin+ Cotrimoxazol, Cotrimoxazole + Tetracycline and Chloramphenicol + Cotrimoxazol), there were synergistic interactions with all combinations but the highest synergistic interactions was observed in Cotrimoxazol + Amoxicillin (50%) and Cotrimoxazol + Augmentin (70%) combinations, this might be due to the same reasons as in case of tetracycline + amoxicillin and tetracycline + augmentin. This also support the findings of [14] , who reported that combinations of the beta-lactam antibiotic helps other antibiotic penetrate the outer membrane of gram-negative bacteria by cell wall synthesis and there by increases the permeability of the bacterium to other classes of antibiotics thus leading to a synergistic effect in the in vitro studies. This same scenario was observed in Chloramphenicol combination with other antibiotics and may be due to the same reason.
5. Conclusion
Poultry droppings is a potential source of human pathogenic bacteria, multiple antibiotic resistance bacteria observed in this study were of great treat to man as this may cause the treatment of infection caused by these bacteria to be difficult. However, combination therapy is an option to treat these infections, and in vitro experience is needed for the development of future clinical studies, combination of beta-lactam antibiotic with fluoroqunolones, tetracycline, chloramphenicol and cotrimoxazole is synergetic and this will reduce dose related toxicity and prevent resistance to single antibiotic.
Cite this paper
Omoya, F.O. and Ajayi, K.O. (2016) Synergistic Effect of Combined Antibiotics against Some Selected Multidrug Resistant Human Pathogenic Bacteria Isolated from Poultry Droppings in Akure, Nigeria. Advances in Microbiology, 6, 1075-1090. http://dx.doi.org/10.4236/aim.2016.614100
References
- 1. Apata, D.F. (2009) Antibiotic Resistance in Poultry. International Journal of Poultry Science, 8, 404-408.
https://doi.org/10.3923/ijps.2009.404.408 - 2. Collignon, P. (2009) Resistant Escherichia coli—We Are What We Eat. Clinical Infectious Diseases, 49, 202-204.
https://doi.org/10.1086/599831 - 3. Hammerum, A.M. and Heuer, O.E. (2009) Human Health Hazards from Antimicrobial-Resistant Escherichia coli of Animal Origin. Clinical Infectious Diseases, 48, 916-921.
https://doi.org/10.1086/597292 - 4. Lister, P.D. (2006) The Role of Pharmacodynamic Research in the Assessment and Development of New Antibacterial Drugs. Biochemical Pharmacology, 7, 1057-1065.
https://doi.org/10.1016/j.bcp.2005.10.038 - 5. Mathew, A.G., Cissell, R. and Liamthong, S. (2007) Antibiotic Resistance in Bacteria Associated with Food Animals: A United States Perspective of Livestock Production. Foodborne Pathogens and Disease, 4, 115-133.
https://doi.org/10.1089/fpd.2006.0066 - 6. Caprioli, A., Busani, L., Martel, J.L. and Helmuth, R. (2000) Monitoring of Antibiotic Resistance in Bacteria of Animal Origin: Epidemiological and Microbiological Methodologies. International Journal of Antimicrobial Agents, 14, 291-294.
https://doi.org/10.1016/S0924-8579(00)00139-4 - 7. Kolár, M., Pantucek, R., Bardoň, J., Vágnerová, I., Typovská, H., Válka, I. and Doskar, J. (2002) Occurrence of Antibiotic-Resistant Bacterial Strains Isolated in Poultry. Veterinary Medicine Czech, 47, 52-59.
- 8. Raghunath, D. (2008) Emerging Antibiotic Resistance in Bacteria with Special Reference to India. Journal of Bioscience, 33, 593-603.
https://doi.org/10.1007/s12038-008-0077-9 - 9. Pednekar, P., Jain, R. and Mahajan, G. (2011) Anti-Infective Potential of Hot-Spring Bacteria. Journal of Global Infectious Diseases, 3, 241-245.
https://doi.org/10.4103/0974-777X.83529 - 10. Cotteral and Wiezdowski, J. (2007) Combination Drugs, an Emerging Option for Antibacterial Therapy. Trends in Biotechnology, 25, 547-555.
https://doi.org/10.1016/j.tibtech.2007.09.004 - 11. Olajuyigbe, O.O. (2012) Synergistic Influence of Tetracycline on the Antibacterial Activities of Amoxicillin against Resistant Bacteria. Journal of Pharmacy and Allied Health Sciences, 2, 12-20.
https://doi.org/10.3923/jpahs.2012.12.20 - 12. Margaret, S., et al. (1986) Phenotypic Comparison of Pseudomonas aeruginosa Strains Isolated from a Variety of Clinical Sites. Journal of clinical microbiology, 24, 260-264.
- 13. Vishwanatha, T., et al. (2011) Antibiotic Synergy Test: Checkerboard Method on Multidrug Resistant Pseudomonas aeruginosa. International Research Journal of Pharmacy, 2, 196-198.
- 14. Haidari, F., Rashidi, M.R., Keshavarz, S.A., Mahboob, S.A., Eshraghian, M.R. and Shahi, M.M. (2008) Effects of Onion on Serum Uric Acid Levels and Hepatic Xanthine Dehydrogenase/Xanthine Oxidase Activities in Hyperuricemic Rats. Pakistan Journal of Biological Science, 11, 1779-1784.
https://doi.org/10.3923/pjbs.2008.1779.1784 - 15. Rashidul Bari, A.H.M., Zafrul Azam, A.T.M., Amran, M.S. and Hossain, M.A. (2000) In Vivo Effects of Ibuprofen and Naproxen on the Plasma Concentration of Diltiazem in Rabbits. Pakistan Journal of Biological Science, 3, 555-557.
https://doi.org/10.3923/pjbs.2000.555.557 - 16. Woodford, N. and Livermore, D.M. (2009) Infections Caused by Gram-Positive Bacteria: A Review of the Global Challenge. Journal of Infection, 59, S4-S16.
https://doi.org/10.1016/S0163-4453(09)60003-7 - 17. Adegunloye, D.V. (2006) Microorganisms Associated with Poultry Faeces. Journal of Food, Agriculture and Environment, 4, 41-42.
- 18. Siwela, A.H., Matsaure, F., Ncube, T., Olonitola, O.S. and Best, G.R. (2007) A Comparison of Antibiotic Resistance in Microorganisms Isolated from Chicken and Ostrich Faeces in Bulawayo, Zimbabwe. International Journal of Biological and Chemical Science, 1, 158-164.
- 19. Fawole, M.O. and Oso, B.A. (2004) Characterization of Bacteria: Laboratory Manual of Microbiology. 4th Edition, Spectrum Book Ltd., Ibadan, 24-33.
- 20. Clinical and Laboratory Standards Institute (CLSI) (2014) Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI Document M100-S24, Wayne, 34(1).
- 21. Hamouda, A.M. and Elbanna, H.I.R. (2013) Combined Antimicrobial Effect against Some Isolated Bacteria from Chickens. Journal of Physiology and Pharmacology Advances, 3, 272-276.
- 22. Eliopoulos, G.M. and Moellering, R.C. (1991) Antimicrobial Combination. In: Lorian, V., Ed., Antibiotics in Laboratory Medicine, Williams and Wilkins, Baltimore, 432-492.
- 23. Eiman, A.S. (2009) The Synergistic Effect of Some Antibiotics against Clinical Isolates of Pseudomonas aeruginosa. Basrah Journal of Veterinary Research, 8, 110-119.
- 24. Collee, J.G., Fraser, A.G., Marmion, B.P. and Simmons, A. (1996) Mackie and McCarteny Practical Medical Microbiology. 14th Edition, Churchill Livingstone.
- 25. Omojowo, F. and Omojasola, F. (2013) Antibiotic Resistance Pattern of Bacterial Pathogens Isolated from Poultry Manure Used to Fertilize Fish Ponds in New Bussa, Nigeria. Albanian Journal of Agricultural Science, 12, 81-85.
- 26. Arifatun, N., Mashuk, S., Shamsun, N., Kazi, S.A., Sk, I.A. and Salequl, I. (2014) Multidrug Resistant-Proteus Mirabilis Isolated from Chicken Droppings in Commercial Poultry Farms: Bio-Security Concern and Emerging Public Health Threat in Bangladesh. Journal of Biosafety and Health Education, 2, 120.
- 27. Mbata, I. (2007) Poultry Meat Pathogens and Its Control. Internet Journal of Food Safety, 7, 20-28.
- 28. Cloud, S.S., Rosenberger, J.K., Fries, P.A., Wilson, R.A. and Odor, E.M. (1985) In Vitro and in Vivo Characterization of Avian Escherichia coli. I. Serotypes, Metabolic Activity and Antibiotic Sensitivity. Avian Diseases, 29, 1084-1093.
https://doi.org/10.2307/1590463 - 29. Muhammad, M., Muhammad, L.U., Ambali, A.G. and Mani, A.U. (2010) A Survey of Early Chick Mortality on Small-Scale Poultry Farms in Jos, Central Nigeria. International Journal of Poultry Science., 9, 446-449.
https://doi.org/10.3923/ijps.2010.446.449 - 30. Hemen, J.T., Johnson, J.T., Ambo, E.E., Ekam, V.S., Odey, M.O. and Fila, W.A. (2012) Multi-Antibiotic Resistance of Some Gram Negative Bacterial Isolates from Poultry Litters of Selected Farms in Benue State. International Journal of Science and Technology, 2, 543-547.
- 31. Mohammad, M.S.D., et al. (2009) Characterization of Antibiotic Resistant Patterns of Salmonella Serotypes Isolated from Beef and Chicken Samples in Tehran. Jundishapur Journal of Microbiology, 2, 124-131.
- 32. Salam, M.A., et al. (2009) In Vitro and in Vivo Effects of Glipizide and Gliclazide on the Protein Binding, Plasma Concentration and Serum Glucose, Cholesterol and Creatinine Levels of Ibuprofen. Journal of Pharmacology and Toxicology, 4, 307-313.
https://doi.org/10.3923/jpt.2009.307.313 - 33. Sato, Y., et al. (2004) Variation in Synergistic Activity by Flavone and Its Related Compounds on the Increased Susceptibility of Various Strains of Methicillin-Resistant Staphylococcus aureus to β-Lactam Antibiotics. International Journal of Antimicrobial Agents, 24, 226-233.
https://doi.org/10.1016/j.ijantimicag.2004.02.028