American Journal of Analytical Chemistry
Vol.05 No.16(2014), Article ID:51722,12 pages
10.4236/ajac.2014.516120
Extending Functionality of Microalgae and Transesterification under Supercritical Fluid Conditions
Chamil A. Biktach, Rustem A. Usmanov, Farid M. Gumerov, Zufar I. Zaripov, Farizan R. Gabitov, Vener F. Khayrutdinov, Ilmutdin M. Abdulagatov
Federal State Budgetary Educational Institution of Higher Professional Education “Kazan National Research Technological University”, Kazan, Russia
Email: xatyaz@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 16 September 2014; revised 31 October 2014; accepted 16 November 2014
ABSTRACT
The experimental data on supercritical CO2 extraction of microalgae are presented. It is confirmed that microalgae contains omega-3 fatty acid components. Phase equilibria data are presented for the triolein-methanol (T = 413 K, P = 5.8 - 11.9 MPa) and ethyl eicosapentaenoate-carbon dioxide (T = 313 - 333 K, P = 10 - 21 MPa) binary systems. The scheme of the batch-type experimental setup for supercritical transesterification of oils is presented. Temperature and molar ratio dependences of non-refined palm oil yield to fatty acid methyl esters (FAME) are presented for T = 563 - 693 K, methanol to palm oil molar ratio 39:1. Experiments on ultrasonic emulsification of rapeseed oil-ethanol mixture (molar ratios 150:1 - 7:1) were conducted. Research data on ultrasonic emulsion stability are presented for the time range of 0 - 40 minutes after ultrasonication completion. Correlation is defined between FAME yield of emulsified reaction mixtures and the emulsion grain size. FAME yields are compared for emulsified and non-emulsified reaction mixtures.
Keywords:
Supercritical methanol, transesterification, biodiesel, microalgae, supercritical fluid, supercritical carbon dioxide

1. Introduction
Biodiesel fuel on the markets of developed countries is produced mainly by conventional catalytic transesterification. However this method is sensitive to water and free fatty acids content in feedstock [1] requiring further removal of catalyst and saponification product remains from biodiesel and waste water utilization [2] .
Supercritical fluid transesterification lacks the abovementioned drawbacks, however its commercialization is hindered with high energy consumption to bring process temperature and pressure to reaction conditions above the critical point (Tc = 513 K, Pc = 8.1 MPa for methanol). To reach higher reaction yield, significant excess of alcohol is needed [3] , (Figure 1), the theoretical methanol-to-oil molar ratio being 3:1.
Other factors undermining biodiesel production cost include high feedstock cost (crops need to be grown, oil, extracted) and competition in arable land use with food and forage crops [4] .
As the abovesaid, it stipulates the need to find ways to lower presently severe transesterification reaction conditions and select efficient feedstock for transesterification process.
Possible ways to decrease process temperature and pressure include selecting optimal molar ratio of reagents and ultrasonic emulsification of reaction mixture.
In terms of sustainable development of global economy, virtually the only feedstock enabling biodiesel production to the tune of completely substituting fossil diesel is microalgae [5] . It contains up to 80% oil (dry mass) [6] [7] , the average value being 20% - 50% [5] . Microalgae crop yield exceeds that of conventional oil crops by an order of magnitude, the crops being harvestable repeatedly and even continuously during year.
Apart from being an energy source, microalgae are also the source of valuable chemicals, namely, omega-3 and omega-6 fatty acids that are widespread as dietary supplements improving health [8] [9] and fighting diseases [10] .
Valuable components of microalgae can be extracted with supercritical CO2 [17] - [19] which, unlike hexane, can provide high purity of the extracted product and extraction selectivity [20] [21] .
In this work, research was done in several aspects. Supercritical CO2 extraction of microalgae was done with consecutive mass-spectometry analysis of the components. Transesterification yield of palm oil-methanol was studied at various reaction parameters which were supplemented by data on stability of ultrasonic emulsions of oils and alcohols.
2. Experimental
2.1. Microalgae drying and extraction
In [22] we conducted supercritical CO2 extraction of microalgae (nannochloropsis salina) and analyzed the extracts. Atmospheric and vacuum drying was conducted on the setup described in [23] during 14 - 16 hours which resulted in humidity decrease from 68.63% to 3.88%. The microalgae sheets obtained were ground to 0.5 - 1 mm and loaded to the extraction cell of the experimental setup (Figure 2). Extraction was conducted at 313 K, 35 MPa. Extract samples weighing 0.14 - 0.62 g. underwent chromatography-mass-spectrometry analysis at DFS mass- spectrometer [17] . Analysis parameters were as follows: injector temperature 553 K (exposure time 60 min), thermostat initial temperature 393 K, heating rate 6 K/min; carrier gas-helium, flow rate 1 ml/min, flow splitting 1:10; capillary column with immobilized nonpolar phase type DB-5.30 m/0.25 mm/0.25 μ. Electron ionization mass-spectra were registered in the 100 - 1000 amu mass range. Spectrometer mass adjustment was carried out using Autotune OEM software. Component identification was done using NIST-05 specialized mass-spectra library.
2.2. Experimental setup for studying phase equilibria of triolein-methanol and ethyl eicosapentaenoate (ethyl-EPA)-CO2 binary systems
Vegetable oils mainly consist of triglycerides of saturated and unsaturated fatty acids. In terms of process modelling, certain stages of proposed biodiesel production flow require reliable phase equilibrium data of oil in the media for supercritical extraction (CO2) and supercritical transesterification (methanol).
To study phase equilibria of the triolein-methanol and ethyl-EPA-CO2 binary systems, following the description provided in [24] we built the experimental setup described in Figure 3 equipping it with the P-50 metering pumps supplied by Thar SFC.
The phase equilibrium cell was evacuated, then filled with the solute (triolein or ethyl-EPA). After that the solvent (methanol or CO2) was fed with themetering pump to the experimental pressure and the equilibrium cell was heated to the experimental temperature. After 60 min stirring the equilibrium cell was put to rest vertically for 90 min.
Figure 1. Literature data on temperature dependence of reation yield of non-refined palm oil to FAME at methanol-to-oil molar ratio of 11 - 60 and reaction time of 20 - 50 min, 20 min, 28:1 [11] [12] ; 20 min, 50:1 [13] ; 30 min, 25:1 [11] ; 40 min, 11:1 [13] - [15] , 40 min, 22:1 [11] [13] [15] ; 40 min, 60:1 [15] ; 50 min, 24.5:1 [11] [16] ; 50 min, 45:1 [16] .
Figure 2. Extraction unit scheme. 1: CO2 cylinder; 2, 8, 10: valves; 3: filter drain 4: cooler; 5: thermostat; 6: CO2 pump; 7: solvent pump; 9: extraction cell; 11: sample tank; 12: solvent tank.
Figure 3. Experimental phase equilibrium setup. 1: methanol tank/CO2 cylinder; 2: high pressure metering pump; 3: phase equilibrium cell with swing; 4: vacuum pump; 5: separating settler (volumetric tube); 6: triolein/ethyl-EPA tank; 7: heating circuit voltage transformer; 8: manometer; 9: temperature regulator; 10, 11, 12, 13: high pressure valves.
The upper phase of triolein-methanol system was collected using syringe. The sample volume Vsam. and the volume of the triolein Vtr. separated to the bottom phase of the sample were measured with a 5-ml volumetric tube.
Triolein volume concentration:
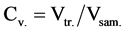 (1)
(1)
Triolein mass concentration:
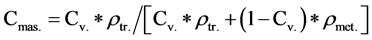 (2)
(2)
where ρtr. and ρmet. are the triolein and methanol densities.
Triolein molar concentration:
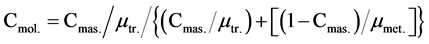 (3)
(3)
where μtr. and μmet. are the triolein and methanol molar masses.
Triolein, essentially thealmond oil supplied by Aspera Ltd. (Moscow) had the purity of 90%, the rest falling to trilinolenin and negligible amount of vitamins and proteins. 99% pure methanol was supplied by Kazanorgsintez JSC.
In the ethyl-EPA-CO2 system the upper phase was collected to a preliminarily weighed balloon submersed in water. Sample volume was defined as the volume of displaced water. After phase separation was complete inside the balloon, it was wiped dry and weighed. The mass of ethyl-EPA dissolved in CO2 was defined as the mass difference of empty balloon and balloon with ethyl-EPA.
CO2 mass concentration in the upper phase is defined as:
 (4)
(4)
where  is the carbon dioxide density inside the balloon, Vatm is the volume of carbon dioxide under pressure inside the balloon [25] .
is the carbon dioxide density inside the balloon, Vatm is the volume of carbon dioxide under pressure inside the balloon [25] .
Molar concentration of CO2 in the upper phase is defined as:
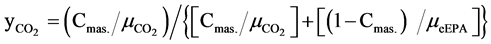 (5)
(5)
where 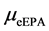 and
and 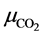 are the molar masses of ethyl EPA and CO2.
are the molar masses of ethyl EPA and CO2.
Ethyl EPA supplied by takeomega3.com was 90% pure, the remaining 10% falling to ethyl docosahexaenoate and tocopherols. 99.5% pure carbon dioxide was supplied by OOO TehGaz.
2.3. Batch-type experimental non-catalytic transesterification setup
32-ml batch-type experimental setup was created [26] designed for 60 MPa pressure and 800 K temperature (Figure 4).
The transesterification reaction is conducted in autoclave 1 that is isochorically heated by a muffle furnace 2 to the experimental temperature and pressure. Mounted on a hinge, the furnace can be rotated about horizontal axis to provide stirring inside the high-pressure autoclave. Temperature is measured with chromel-alumel thermocouple whose thermojunction is located centrally inside the autoclave. The thermocouple is preliminarily calibrated with the PTS-10 platinum resistance thermometer. Measurement accuracy is ±5 K. Pressure is measured with Stanley-Korund DI-001 pressure sensor 3 powered by the direct current power supply 5. Pressure and temperature measurements are displayed on TRM-101 indicating gauges 6. Process pressure can be adjusted with the VK-97 high pressure needle valve 4.
The reaction autoclave is 500 mm long, cylinder-shaped, with the external diameter of 26 mm, internal diameter of 12 mm (Figure 5).
Figure 4. Experimental transesterification setup scheme.
Figure 5. Reaction autoclave for supercritical transesterification: 1: thermo- couple; 2: to pressure sensor; 3: olive; 4: body; 5: flanges; 7: bolt joint.
The setup embodies the transesterification process designed by Saka and Kusdiana [27] . Transesterification experiments were done with the non-refined palm oil.
Reaction products separation and composition analysis are described in [26] .
2.4. Reaction mixture emulsification and technique for studying emulsion properties
In this work the emulsion of initial reagents was obtained using 24 kHz, 200 W Hielscher Ultrasonic UIP200 hd ultrasonic emulsifier [28] .
Emulsion stability by time was assessed visually, by the amount of oil separating from the emulsion inside measuring tank. We called the share of oil that separated from the emulsion as emulsion layering coefficient.
3. Results and Discussion
3.1. Microalgae Extraction in Supercritical Carbon Dioxide
Each of the samples was found out to be a multicomponent mixture consisting primarily of triglycerides and esters of saturated and unsaturated fatty acids. Composition of one of the samples is show in the chromatogram (Figure 6) and in Table 1.
3.2. Phase equilibria of triolein-methanol and ethyl-EPA-CO2 systems
Experimental phase equilibrium data for the triolein-methanol system at 413 K and various pressures are presented in Figure 7.
The phase equilibrium diagram is in satisfactory agreement with the literature data [29] . There seems to be discrepancy at 10 MPa, however this and neighboring isotherms of the same literature work altogether fail to represent a data array without unexplained anomalies which can mean significant measurement error in this measurement scale. Anyway the research has proved very low solubility in sub-critical area of triolein, one of the main components of vegetable oils, in methanol, the other component of the transesterification reaction, which is a significant limiting factor for the reaction.
Phase equilibrium was also studied for the ethyl eicosapentaenoate (ethyl-EPA)-CO2 system at 313, 323 and 333 K in the 10 - 21 MPa pressure range. Experimental data are presented in Figures 8-10.
Experimental data agree satisfactorily with the literature data, while discrepancies may be explained by differences in the composition of substances studied. In all figures, the experimental curve tends to horizontal, as pressure grows, which can mean full solubility of the components.
Maximum error in measuring phase equilibrium characteristics was calculated as 9.7%.
3.3. Dependence of supercritical transesterification reaction yield on reaction mixture composition and thermodynamic parameters
Experiments on obtaining biodiesel fuel in batch-type setup were conducted in the 563 - 793 K temperature range,
Table 1. Chromatography-mass-spectrometry analysis results of the sample No. 3.
Ethyl 5, 8, 11, 14, 17-eicosapentaenoate, an omega-3 component was found among extracted substances in quantity of 1.75%.
Figure 6. Full chromatogram of the sample No. 3 and the closeup with the ethyl eicosapentaenoate (peak 28).
Figure 7. Triolein-methanol phase equilibrium at 413 K. 1: experimental. 2: [29] .
Figure 8. Ethyl-EPA-CO2 phase equilibrium at 313 K. Hori- zontal axis: CO2 molar concentration; vertical axis: pressure, MPa. 93% ethyl-EPA-experimental. 90% ethyl-EPA [30] .
Figure 9. Ethyl-EPA-CO2 phase equilibrium at 323 K. Hori- zontal axis: CO2 molar concentration; vertical axis: pressure, MPa. 93% ethyl-EPA-experimental. 90% ethyl-EPA [30] .
Figure 10. Ethyl-EPA-CO2 phase equilibrium at 333 K. Hori- zontal axis: CO2 molar concentration; vertical axis: pressure, MPa. 93% ethyl-EPA-experimental. 90% ethyl-EPA [30] .
19.7 - 44 MPa pressure range, with methanol-to-oil molar ratios of 304:1 to 6:1.
Non-refined palm oil produced by Seloga Petroleum (Malaysia) from elaeis guineensis crop in 2007 had the following characteristics: humidity and impurities content < 0.1%, free fatty acids < 0.1%, iodine number 50, cloud point 283 K, peroxide number < 1. Refined palm oil branded Zlata Palma had the following characteristics: free fatty acids content < 0.1%, humidity < 0.1%, iodine number 64, cloud point 277 K, saturated fatty acid components 39.7%, monounsaturated fatty acid components 46%, polyunsaturated fatty acids 14.2%. 99% methanol was supplied by Kazanorgsintez JSC.
Reaction yield was defined as:

where Vrme and Vmet are the volumes of the raw methyl esters phase and the methanol phase in the sample after reaction.
Figure 11 and Figure 12 compare the experimental data for FAME yield at 10 min reaction time at various temperatures and methanol-to-oil molar ratios to our previously published results [26] and literature data [11] [12]
Figure 11. Temperature dependence of FAME yield for non- refined palm oil, 10 min reaction time, molar ratios of 37:1 compared to literature data. 231:1, 168:1, 104:1 [26] (our pre- vious work); 41:1 [31] [32] ; 39:1 experimental (present work); 27:1 [11] [12] ; 18:1, 12:1 [33] .
Figure 12. Methanol-to-oil molar ratio dependence of FAME yield for non-refined palm oil, temperatures of 567 - 690 K compared with literature data. 568 K, 610 K, 638 K [26] (our previous work); 567 K [13] [31] ; 673 K [13] [33] ; 690 K experimental (present work).
[31] - [33] .
Figure 13 compares experimental data on the FAME yield of refined palm oil at 10 min reaction time at various temperatures and methanol-to-oil molar ratios with our previously published results [26] and literature data [34] .
Figure 14 features the pressure dependence of reaction yield at constant temperature and molar ratio.
Dependences presented are in qualitative agreement with those in [3] [16] [35] and [36] . At pressures above 20 MPa the influence of temperature on the reaction yield was shown to be more significant than that of pressure which is congruent with the literature results [36] - [38] .
Calculated maximum error in measuring FAME yield is 2.27%.
3.4. Initial reaction mixture emulsion stability and grain size dependence of reaction rate
Experimental data are presented on phase separation dynamics of ultrasonicated emulsions of ethanol and rapeseed oil. Rapeseed oil provided by OAO Nefis-Cosmetics was produced from brassica napus (Tatarstan, harvested in 2008) by GOST 1129-93, acid number < 3, humidity and volatile components content < 0.1%, negligible amount of FAME present. 99% pure ethanol was supplied by Kazanorgsintez JSC.
Emulsion examined had the ethanol-to-oil molar ratio of 150:1 to 7:1 (oil volume concentrations of 0.1 - 0.7). Ultrasonic treatment time was conducted for 30 - 120 s, the treatment power being 100 - 200 W. Emulsions with the ratio 7:1 (volume concentration 0.7) proved to essentially separate very fast, within 1 - 5 minutes with further slowing of separation while emulsions with the ratio of 17:1 (volume concentration 0.5) and higher remained relatively stable for longer time range and separated slower (Figure 15).
Emulsions obtained were examined under microscope to learn the emulsion grain size. Samples with 2.26 - 8.6 μm grain size (Figure 16) were used in transesterification experiments which proved that fatty acid alkyl ester yield is highest when the grain size is smallest (Figure 17).
Experimental data are presented on temperature dependence of reaction yield for non-refined rapeseed oil and methanol mixtures. Molar ratio was 153:1, reaction time, 10 min. Transesterification reactions were carried out in
Figure 13. Methanol-to-oil molar ratio dependence of FAME yield for refined palm oil, at temperatures of 595 - 685 K com- pared with literature data. 595 K, 685 K experimental; 623 K [15] .
Figure 14. Pressure dependence of FAME yield of non-refined palm oil at reaction time of 10 min, methanol-to-oil molar ratio of 125:1 and reaction temperature 605 K.
Figure 15. Time dependence of non-refined rapeseed oil-etha- nol emulsion layering coefficient at ultrasonication treatment power of 200 W, treatment time 2 min, molar ratios of 7:1 and 17:1.
Figure 16. Emulsion sample, 3 minutes after ultrasonication completion.
the same experimental setup (Figure 4). FAME yields for reaction mixtures with and without preliminary ultrasonication are compared in Figure 18.
Ultrasonication proved to significantly increase FAME yield at relatively low reaction temperatures (563 K) compared to reaction mixture without preliminary ultrasonication. This difference tends to vanish at higher temperatures (633 K).
No clear correlation impact was found of ultrasonication time (30 - 120 s) and power (100 - 200 W) to emulsion properties.
Maximum error in measuring emulsion layering coefficient was calculated as 1.86%.
4. Conclusions
1) New data have been found on phase equilibrium of initial reagents and their mixtures. Available data on triolein’s poor solubility in methanol were confirmed which complied with the empirical rule that polar solvents hardly dissolved nonpolar solutes. New data were found for phase equilibrium of ethyl-EPA-carbon dioxide system.
2) Experimental data were obtained on phase separation dynamics for ultrasonicated rapeseed oil-ethanol mixtures. Practically stable emulsions can be obtained for ethanol-to-oil molar ratios of 17:1 and higher (0.5 volume concentration of oil). Reaction yield of emulsified mixtures is in direct correlation with grain size, more disperse mixtures reacting more willingly. Emulsification proved to significantly increase reaction yield at relatively low temperatures (563 K) compared to non-treated reagents.
3) 32-ml batch-type experimental setup was built designed for alcohol transesterification reactions at temperatures up to 800 K, pressures up to 60 MPa. The setup design lacks internal stirring equipment; instead, stirring and evening heat distribution are done by swinging the whole furnace about horizontal hinge.
Experimental data were obtained on thermodynamic conditions dependence of FAME yield for the methanol transesterification of palm oil conducted at batch-type setup. It was found that high yields were achievable in a tradeoff between methanol excess and heating to high temperatures. It should be noted that transesterification experiments were done without any preliminary treatment like ultrasonication. Reaction yield data are in
Figure 17. Dependence of non-refined rapeseed oil FAME yield on emulsion grain size and alcohol-to-oil molar ratio for ultrasonicated emulsions.
Figure 18. Temperature dependence of FAME yield for non- refined rapeseed oil, 153:1 methanol-to-oil molar ratio, 10 min reaction time.
a satisfactory agreement with the literature.
4) Possibility to produce biodiesel from microalgae oil was confirmed. Microalgae were dried and supercritical CO2 extraction was carried out. Valuable omega-3 fatty acid components (EPA and DHA) were found in the extract which meant the possibility to improve the overall profitability of the biodiesel production process.
Acknowledgements
Present work was done in Kazan National Research Technological University with the financial support from the Ministry of Education and Science of the Russian Federation (Agreement No. 14.574.21.0085; Project ID RFMEFI57414X0085) and the Russian Foundation for Basic Research grant No. 13-03-12078 ofi-m/14.
References
- Ma, F. and Hanna, M.A. (1999) Biodiesel Production: A Review. Bioresource Technology, 70, 1-15. http://dx.doi.org/10.1016/S0960-8524(99)00025-5
- Demirbas, A. (2007) Biodiesel: A Realistic Fuel Alternative for Diesel Engines. Technology & Engineering. Springer- Verlag, London, 208 p.
- Kusdiana, D. and Saka, S. (2001) Methyl Esterification of Free Fatty Acids of Rapeseed Oil as Treated in Supercritical Methanol. Journal of Chemical Engineering of Japan, 34, 383-387. http://dx.doi.org/10.1252/jcej.34.383
- Gao, Y., Gregor, C., Liang, Y., Tang, D. and Tweed, C. (2012) Algae Biodiesel—A Feasibility Report. Chemistry Central Journal, 6, S1. http://dx.doi.org/10.1186/1752-153X-6-S1-S1
- Chisti, Y. (2007) Biodiesel from Microalgae Beats Bioethanol. Trends in Biotechnology, 26, 126-131. http://dx.doi.org/10.1016/j.tibtech.2007.12.002
- Metting, F.B. (1996) Biodiversity and Application of Microalgae. Journal of Industrial Microbiology & Biotechnology, 17, 477-489. http://dx.doi.org/10.1007/BF01574779
- Spolaore, P., Joannis-Cassan, C., Duran, E. and Isambert, A. (2006) Commercial Applications of Microalgae. Journal of Bioscience and Bioengineering, 101, 87-96. http://dx.doi.org/10.1263/jbb.101.87
- Lands, W.E. (1986) Fish, Omega-3 and Human Health. AOCS Publishing, Orlando, 235 p.
- Kinsella, J.E. (1987) Seafoods and Fish Oils in Human Health and Disease. Marcel Dekker Inc., New York, 317 p.
- Scott, D., Srirama, K. and Sanjeevi, P.B. (2007) Omega-3 Fatty Acids for Nutrition and Medicine: Considering Microalgae Oil as a Vegetarian Source of EPA and DHA. Current Diabetes Reviews, 3, 198-203. http://dx.doi.org/10.2174/157339907781368968
- Asri, N.P., Machmudah, S., Wahyudiono, W., Suprapto, S., Budikarjono, K., Roesyadi, A. and Goto, M. (2013) Non Catalytic Transesterification of Vegetables Oil to Biodiesel in Sub- and Supercritical Methanol: A Kinetic’s Study. Bulletin of Chemical Reaction Engineering & Catalysis, 7, 215-223. http://dx.doi.org/10.9767/bcrec.7.3.4060.215-223
- Tan, K.T., Lee, K.T. and Mohamed, A.R. (2009) Production of FAME by Palm Oil Transesterification via Supercritical Methanol Technology. Biomass and Bioenergy, 33, 1096-1099. http://dx.doi.org/10.1016/j.biombioe.2009.04.003
- Rathore, V. and Madras, G. (2007) Synthesis of Biodiesel from Edible and Non-Edible Oils in Supercritical Alcohols and Enzymatic Synthesis in Supercritical Carbon Dioxide. Fuel, 86, 2650-2659. http://dx.doi.org/10.1016/j.fuel.2007.03.014
- Chanchaochai, P., Boonnoun, P., Laosiripojana, N., Goto, M., Jongsomjit, B., Panpranot, J., Mekasuwandumrong, O. and Shotipruk, A. (2013) Transesterification of Palm Oil at Near Critical Conditions Using Sulfonated Carbon-Based Acid Catalyst. Chemical Engineering Communications, 200, 1542-1552. http://dx.doi.org/10.1080/00986445.2012.749249
- Hong, S.T., Kim, J.-W., Jang, W.-H., Lim, J.S., Park, H.S., Yoo, K.-P., Apfel, C. and Arlt, W. (2009) Transesterification of Palm Oil Using Supercritical Methanol with Co-Solvent HCFC-141b. Research on Chemical Intermediates, 35, 197-207. http://dx.doi.org/10.1007/s11164-008-0018-0
- Jomtib, N., Goto, M., Sasaki, M. and Shotipruk, A. (2006) Production of Biodiesel from Palm Oil in Supercritical Methanol. Chulalongkorn University, Bangkok, 24-27.
- Biktashev, S.A., Yarullin, L.Y., Gumerov, F.M., Gabitov, F.R., Usmanov, R.A., Abdulagatov, I.M. and Willson, B. (2011) Extraction of Biologically Active Fatty Acids from Microalgae in Supercritical Carbon Dioxide. Herald of Kazan Technological University, 17, 251-253.
- Temelli, F., LeBlanc, E. and Fu, L. (1995) Supercritical CO2 Extraction of Oil from Atlantic Mackerel (Scomber scombrus) and Protein Functionality. Journal of Food Science, 60, 703-706. http://dx.doi.org/10.1007/s11164-008-0018-0
- Chernyshev, A.K., Gumerov, F.M., Tsvetnitskiy, G.N., Yarullin, R.S., Ivanov, S.V., Levin, B.V., Shafran, M.I., Zhilin, I.F., Beskov, A.G. and Chernyshev, K.A. (2013) Carbon Dioxide: Properties, Collection (Obtaining), Application. Infohim, Moscow, 580-694.
- Brunetti, L., Daghetta, A., Fedell, E., Kikic, I. and Zanderighi, L. (1998) Deacidification of Olive Oils by Supercritical Carbon Dioxide. Journal of the American Oil Chemists’ Society, 66, 209-217. http://dx.doi.org/10.1007/BF02546062
- Molero Gómez, A., Pereyra López, C. and de la Ossa, E.M. (1996) Recovery of Grape Seed Oil by Liquid and Supercritical Carbon Dioxide Extraction: A Comparison with Conventional Solvent Extraction. The Chemical Engineering Journal and the Biochemical Engineering Journal, 61, 227-231. http://dx.doi.org/10.1016/0923-0467(95)03040-9
- Gabitov, F.R., Usmanov, R.A., Yarullin, L.Y., Biktashev, S.A., Gayfullina, R.R. and Maryashev, A.V. (2012) Experimental Study of the Supercritical Extraction of Algae. Herald of Kazan Technological University, 15, 67-69.
- Gayfullina, R.R., Kurbangaleyev, M.S., Madyakin, V.F., Abramov, Y.K., Zaripov, Z.I., Anashkin, D.A. and Hismatov, B.M. (2009) Experimental Setup for Studying Pulsed Vacuum Drying. Russian Nat. Sci.-Tech. and Method. Conf. Proc., 97 p.
- Gumerov, F.M., Sabirzyanov, A.N. and Gumerova, G.I. (2007) Sup- and Supercritical Fluids in Polymer Processing. Fen, Kazan, 334 p.
- Vargaftik, N.B. (1972) Thermophysical Properties of Gases and Liquids, a Reference Book. Nauka, Moscow, 720 p.
- Biktashev, S.A., Usmanov, R.A., Gabitov, R.R., Gazizov, R.A., Gumerov, F.M., Gabitov, F.R., Abdulagatov, I.M., Yarullin, R.S. and Yakushev, I.A. (2011) Transesterification of Rapeseed and Palm Oils in Supercritical Methanol and Ethanol. Biomass and Bioenergy, 35, 2999-3011. http://dx.doi.org/10.1016/j.biombioe.2011.03.038
- Saka, S. and Kusdiana, D. (2001) Biodiesel Fuel from Rapeseed Oil as Prepared in Supercritical Methanol. Fuel, 80, 225-231. http://dx.doi.org/10.1016/S0016-2361(00)00083-1
- Gabitov, R.R., Usmanov, R.A., Gumerov, F.M. and Gabitov, F.R. (2012) Research of Emulsion Stability of Rapeseed Oil—Ethanol Mixture Obtained by Ultrasonic Emulsification. Herald of Kazan Technological University, 7, 129-132.
- Tang, Z., Du, Z., Min, E., Gao, L., Jiang, T. and Han, B. (2006) Phase Equilibria of Methanol—Triolein System at Elevated Temperature and Pressure. Fluid Phase Equilibria, 239, 8-11. http://dx.doi.org/10.1016/j.fluid.2005.10.010
- Bharath, R., Inomata, H., Arai, K. Shoji, K. and Noguchi, Y. (1989) Vapor-Liquid Equilibria for Binary Mixtures of Carbon Dioxide and Fatty Acid Ethyl Esters. Fluid Phase Equilibria, 50, 315-327. http://dx.doi.org/10.1016/0378-3812(89)80298-5
- Petchmala, A., Laosiripojana, N., Jongsomjit, B., Goto, M., Panpranot, J., Mekasuwandumrong, O. and Shotipruk, A. (2010) Transesterification of Palm Oil and Esterification of Palm Fatty Acid in Near- and Super-Critical Methanol with SO4-ZrO2 Catalysts. Fuel, 89, 2387-2392. http://dx.doi.org/10.1016/j.fuel.2010.04.010
- Tan, K.T., Lee, K.T. and Mohamed, A.R. (2011) Potential of Waste Palm Cooking Oil for Catalyst-Free Biodiesel Production. Energy, 36, 2085-2088. http://dx.doi.org/10.1016/j.energy.2010.05.003
- Sawangkeaw, R., Teeravitud, S., Bunyakiat, K. and Ngamprasertsith, S. (2011) Biofuel Production from Palm Oil with Supercritical Alcohols: Effects of the Alcohol to Oil Molar Ratios on the Biofuel Chemical Composition and Properties. Bioresource Technology, 102, 10704-10710. http://dx.doi.org/10.1016/j.biortech.2011.08.105
- Song, E.-S., Lim, J., Lee, H.-S. and Lee, Y.-W. (2008) Transesterification of RBD Palm Oil Using Supercritical Methanol. The Journal of Supercritical Fluids, 44, 356-363. http://dx.doi.org/10.1016/j.supflu.2007.09.010
- Kogan, V.B., Fridman, V.M. and Kafarov, V.V. (1961) “Binary Systems” in Solubility Reference Book. USSR Academy of Science Press, Moscow-Leningrad, 970 p.
- Choi, C.-S., Kim, J.-W., Jeong, C.-J., Kim, H. and Yoo, K.-P. (2011) Transesterification Kinetics of Palm Olein Oil Using Supercritical Methanol. The Journal of Supercritical Fluids, 58, 365-370. http://dx.doi.org/10.1016/j.supflu.2011.06.015
- Sawangkeaw, R., Tejvirat, P., Ngamcharassrivichai, C. and Ngamprasertsith, C. (2012) Supercritical Transesterification of Palm Oil and Hydrated Ethanol in a Fixed Bed Reactor with a CaO/Al2O3 Catalyst. Energies, 5, 1062-1080. http://dx.doi.org/10.3390/en5041062
- Micic, R.D., Tomić, M.D., Kiss, F.E., Nikolić-Djorić, E.B. and Simikić, M.Ð. (2014) Influence of Reaction Conditions and Type of Alcohol on Biodiesel Yields and Process Economics of Supercritical Transesterification. Energy Conversion and Management, 86, 717-726.




















