Journal of Materials Science and Chemical Engineering
Vol.05 No.01(2017), Article ID:73249,7 pages
10.4236/msce.2017.51001
Template-Free Bipotentiostatic Deposition of Thermoelectric BixTey Nano Arrays
Xin Bo1,2, Feng Wang2, Chuan Zhao1
1School of Chemistry, the University of New South Wales, Sydney, Australia
2State Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Electrochemical Process and Technology for Materials, Beijing University of Chemical Technology, Beijing, China




Received: October 14, 2016; Accepted: January 1, 2017; Published: January 4, 2017
ABSTRACT
Monodispersed Bi-Tenano arrays are achieved via template-free bipotentiostatic deposition. The diameter and length of individual nanorod is ~80 nm and ~250 nm respectively. The electrodeposition process is demonstrated to follow a two-step mechanism: an instantaneous reductive potential is applied to form dispersive nuclei, then a reverse oxidative potential strips partial Bi atoms to prevent further cross-growth. Repeatedly, the nano arrays film is obtained eventually. The thermoelectric properties of the obtained Bi-Tenano arrays such as electrical resistance, carrier density, Seebeck coefficient and power factor are measured to be 2.438 × 10−4 Ω・m, 4.251 × 1020 cm−3, −25.892 μV・K−1, 2.750 × 10−6 W・m−1・K2, respectively.
Keywords:
Thermoelectric Materials, Bismuth Telluride, Nano Arrays, Template-Free, Electrodeposition

1. Introduction
Thermoelectric (TE) materials have stimulated significant renewed interest recently because of their applications in eco-friendly energy conversion [1]. These materials are promising for power generators to transfer waste heat into electricity directly [2]. However, the application of thermoelectric materials is currently limited due to its poor efficiency. Therefore it is necessary to design a new type of materials with enhanced TE performance. Conventionally, the performance of bulk thermoelectric materials is generally evaluated by the dimensionless figure of merit ZT (ZT = S2σTκ−1), where S is the thermoelectric Seebeck coefficient, σ represents for electric conductivity and κ for thermal conductivity [2].
Bi2Te3 has been widely regarded as an excellent candidate for its rhombohedral-layered structure, which is described as hexagonal symmetry and anisotropic properties [3]. Due to its unique layered structure, Bi2Te3 exhibits high values of thermoelectric Seebeck coefficient and electric conductivity. Paradoxically, the increase of the electric conductivity leads to the rise of thermal conductivity, which would negatively influence on ZT value. Alternatively, quantum confinement provides with a feasible way to improve ZT value on the other hand. According to the equation of κ = κe + κp, where κe and κp represent the electrical thermal resistance and the phonon thermal resistance, it is possible to only lower the κp part without disturbing the electrical property by low-di- mensional design such as thin film and nanorod/wire because the strong lattice scattering can hinder the thermal phonon broadcast through the lattice and grain boundary [4]. Particularly, the performance of lower-dimensional TE materials is evaluated by Power Factor (PF = S2σ).
Thin film TE materials have been generally achieved by various methods such as chemical/physical vapor deposition [5] [6] and electrodeposition [7] [8]. However to fabricate the one-dimensional materials, novel approaches need to be developed. The nanorod/wire with more complicated structure could be obtained in aqueous reaction but the samples were dispersed in the solution, which cannot be directly characterized or used without modeling into bulk materials [9]. When it comes to galvanic replacement [10], only elemental thermoelectric material like Te0 could be obtained. As to fabricate large-scale alloy nanorod array film, electrodeposition could be a promising approach. By the use of anodic aluminum oxide template (AAO), the nanorods and wires are uniformly grown on substrates [11]. The insulated AAO template can support and separate the nanorods, however the structure would collapse after removal of the template.
In this paper, we show another approach to achieve large area (1.5 cm × 1.5 cm) thermoelectric Te rich Bi2Te3 nanoarrays via bipotentiostatic deposition without using template. The obtained Bi-Tenano arrays have been characterized in detail by X-ray diffraction patterns (XRD), energy dispersive spectroscopy (EDS), FESEM (field emission scanning electron microscope) and electrochemical methods.
2. Experimental Details
2.1. Electrochemical Testing and Electrodeposition
TeO2 (99.99%, Sinopharm Chemical Reagent Co., Ltd, Beijing, China) and Bi(NO3)3・5H2O (>99.7%, Sinopharm Chemical Reagent Co., Ltd., Beijing, China) were dissolved in the concentrated nitric acid (Sinopharm Chemical Reagent Co., Ltd., Beijing, China) separately and mixed together to obtain the binary solution (15 mM 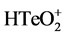 and 10 mM Bi3+). The concentration of nitric acid was then adjusted to 1 M with deionized water. The solution was bubbled in nitrogen gas for 30 min. All chemical agents were directly used without any purification.
and 10 mM Bi3+). The concentration of nitric acid was then adjusted to 1 M with deionized water. The solution was bubbled in nitrogen gas for 30 min. All chemical agents were directly used without any purification.
The electrochemical testings and electrodeposition (chronoamperometry) were carried out with a Princeton 2273 electrochemical working station at room temperature. A standard 3-elecctrode system was introduced, in which a Pt Plate (2.5 cm × 2.5 cm) was used as the counter electrode and the saturated calomel electrode (SCE) as the reference electrode. The working electrode was Indium tin oxide doped SiO2 glass (ITO, 1.5 cm × 1.5 cm) which was subsequently rinsed with acetone, deionized water and ethanol in ultrasonic environment for 15 min, then sputtered with a Au seed layer via ion sputtering device (2 mA, 10 s, SBC-12, KYKY Technology Co., LTD). The pre-treated substrate thereafter was bi-electrodeposited under the periodical applied potentials of −0.20 V/0.20 V and lasting time of 1 s/2 s respectively for 1000 cycles. The linear sweep voltammetry (LSV, 1 mV・s−1) and cyclic voltammetry (CV, 10 mV・s−1) were applied to investigate the electrochemical behavior in metallic aqueous solution.
2.2. Characterization
The obtained Bi-Tenanoarrays/films were analyzed by X-ray diffraction (XRD, Dmax2, Rigaku) with a scanning rate of 4˚ min−1 at 40 kV and 30 mA using Cu Kα as the radiation. The scanning electron microscopy (SEM, FE-JSM 6702F, JEOL) was used to investigate the morphology of the surfaces and cross-sec- tions/thickness of the films. The content of Bi-Te film was measured by energy dispersive spectroscopy (EDS, INCA-Penta FET-X3, Oxford). Then the fabricated nanrod arrays/films were transferred from the conductive substrate to an insulated matrix (unsaturated resins with 5% curing agent) to test the properties [12]. The electric resistance was evaluated by HALL-Effect measuring system (RH2030, Phystech) via a 4-point probe method. The Seebeck coefficient was measured by ZEM-3 device (M8, ULBAC-RIKO), and the applied temperature gradient is controlled from 10˚C, 15˚C and 20˚C. All these properties were measured alone in plane direction.
3. Results and Discussion
Shown in Figures 1(a)-(c), SEM study suggests the nano arrays films were successfully achieved and dispersed uniformly on the substrate. Comparing with thin film achieved at −0.20 V for 120 s, it shows that the morphology is transferred from polycrystalline wheat-like structure into freestanding nanorod arrays. The diameter of the nanorod and length are ~80 nm and ~250 nm respectively. The electrical resistance, carrier density, Seebeck coefficient and power factor of the obtained film are 2.438 × 10−4 Ω・m, 4.25 × 1020 cm−3, −25.89 μV・K−1, 2.75 × 10−6 Wm−1・K2, respectively.
The schematic diagram of bi-potentiostatic deposition process is shown in Figure 2. A negative potential of −0.20 V was applied to form the Bi-Te nuclei. Then a positive potential of 0.20 V was engaged to start a stripping process. Under particular circumstance of the oxidative stripping, the Bi content in the nuclei was ionized again while Te atoms still remain at the lattice sites of Bi2Te3. This is evidenced by the cyclic voltammetry shown in Figure 3, where only one obvious anodic oxide peak in Bi3+ system is observed around the applied potential
Figure 1. SEM images of nano arrays (a)-(c) and thin film (d)-(f).
Figure 2. Schematic of the bipotentiostatic deposition.
Figure 3. CVs of the  (black dash), BiШ (red dot and dash), and binary system (violet).
(black dash), BiШ (red dot and dash), and binary system (violet).
of 0.20 V, whilst not in 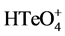 or binary system. Furthermore, the electrochemical corrosion would always begin at the boundary of the nuclei abutting to each other, since this particular region possesses more unbalanced Gibbs free energy than the center of nuclei [13]. For these reasons, the stripping process can be described as that the anodic corrosion is occurred preferentially at the boundary of the nuclei sites where only Bi atoms are dissolved into solution. When repeating the cycles of depositing/stripping procedure, the Bi-Te composites would grow onto the previous point and so forth the nanorod structure is formed.
or binary system. Furthermore, the electrochemical corrosion would always begin at the boundary of the nuclei abutting to each other, since this particular region possesses more unbalanced Gibbs free energy than the center of nuclei [13]. For these reasons, the stripping process can be described as that the anodic corrosion is occurred preferentially at the boundary of the nuclei sites where only Bi atoms are dissolved into solution. When repeating the cycles of depositing/stripping procedure, the Bi-Te composites would grow onto the previous point and so forth the nanorod structure is formed.
To prove the hypothesis of bi-potentiostatic deposition, EDS and XRD were employed. According to EDS (not shown), the atomic ratio of Bi: Te in nanorod structure is 14.15:85.59. Shown in Figure 4, the XRD pattern suggests the nano arrays have the characteristic diffraction peaks of the single phase of Bi2Te3 (JCPDS Card File, 08-0027) except the five significant diffraction peaks from the ITO substrate. Considering the deviation of stoichiometry but single-phase XRD patterns, it is proven that the missing Bi element was dissolved during the stripping anodic process and the rest atoms still stayed at the initial position, which maintained the lattice structure of Bi2Te3. In comparison with the Bi2Te3 single-phase thin film (EDS, Bi:Te = 38.50:61.50) obtained under the applied potential of −0.20 V, the preferred orientation (110) disappears, revealing more defects in nano arrays. Besides, a new plane of (1010) is observed in nano arrays structure. Moreover, there is a consistent positive shift in 2θ values of (110) and (205) planes in nano arrays, proving the lattice contraction in Bi2Te3 according to Bragg’s equation [14]. This phenomenon indicates that the distance between lattice layers becomes smaller due to collapse of lattice structure during Bi-strip- ping process.
Figure 4. XRD patterns of the obtained pure phase thin film (blue), nanorod arrays (red) and Au/ITO substrate (black).
4. Conclusion
Bi-Tenano arrays film has been achieved uniformly on ITO substrate via template-free bi-potentiostatic deposition, which is uniformly dispersed on the ITO substrates. Preferred growing and stripping processes are periodically occurring during the deposition to form the nanorod structure. The TE evaluation of the obtained Bi-Tenano arrays show that the electrical resistance, carrier density, Seebeck coefficient and power factor of nano arrays film are 2.44 × 10−4 Ω・m, 4.25 × 1020 cm−3, −25.89 μV・K−1, 2.75 × 10−6 Wm−1・K2, respectively.
Cite this paper
Bo, X., Wang, F. and Zhao, C. (2017) Template-Free Bipotentiostatic Deposition of Thermoelectric BixTey Nano Arrays. Journal of Materials Science and Chemical Engineering, 5, 1-7. http://dx.doi.org/10.4236/msce.2017.51001
References
- 1. Yoo, B., Xiao, F., Bozhilov, K.N., Herman, J., Ryan, M.A. and Myung, N.V. (2007) Electrodeposition of Thermoelectric Superlattice Nanowires. Adv. Mater., 19, 296- 299. https://doi.org/10.1002/adma.200600606
- 2. Chen, Z.-G., Han, G., Yang, L., Cheng, L. and Zou, J. (2012) Nanostructured Thermoelectric Materials: Current Research and Future Challenge, Progress in Natural Science: Ma-terials International, 22, 535-549. https://doi.org/10.1016/j.pnsc.2012.11.011
- 3. Xiao, F., Hangarter, C., Yoo, B., Rheem, Y., Lee, K.-H. and Myung, N.V. (2008) Recent Progress in Electrodeposition of Thermoelectric Thin Films and Nanostructures. Electrochimica Acta, 53, 8103-8117. https://doi.org/10.1016/j.electacta.2008.06.015
- 4. Yildiz, K., Akgul, U., Leipner, H.S. and Atici, Y. (2013) Electron Microscopy Study of Thermoelectric N-Type Bi2(Te0.9Se0.1)3 Film Deposited by DC Sputtering. Superlattices and Microstructures, 58, 60-71. https://doi.org/10.1016/j.spmi.2013.02.013
- 5. Kang, S.-W., Jeon, K.-M., Shin, J.-S., Chun, J.-R., Kim, Y.-H., Lee, S.J. and Yun, J.-Y. (2013) MOCVD of C-Oriented Bi2Te3 Films on SiO2 Substrates Using Triethyl Bismuth and Di-Tertiarybutyl Tellurium. Chem. Vap. Deposition, 19, 61-67. https://doi.org/10.1002/cvde.201207012
- 6. Deng, Y., Xiang, Y. and Song, Y.Z. (2009) Template-Free Synthesis and Transport Properties of Bi2Te3 Ordered Nanowire Arrays via a Physical Vapor Process. Cryst. Growth Des., 9, 3079-3082. https://doi.org/10.1021/cg800808u
- 7. Yoo, I.-J., Myung, N.V., Lim, D.C., Song, Y., Jeong, Y.-K., Kim, Y.D., Lee, K.H. and Lim, J.-H. (2013) Electrodeposition of BixTey Thin Films for Thermoelectric Application. Thin Solid Films, 546, 48-52. https://doi.org/10.1016/j.tsf.2013.05.036
- 8. Yoo, B.Y., Huang, C.-K., Lim, J.R., Herman, J., Ryan, M.A., Fleurial, J.-P. and Myung, N.V. (2005) Lectrochemically Deposited Thermoelectric N-Type Bi2Te3 Thin Films. Electrochimica Acta, 50, 4371-4377. https://doi.org/10.1016/j.electacta.2005.02.016
- 9. Cheng, L., Chen, Z., Yang, L., Han, G., Xu, H., Snyder, G.J., Lu, G. and Zou, J. (2013) J. Phy. Chem. C., 117, 12458. https://doi.org/10.1021/jp4041666
- 10. Jeong, D.-B., Lim, J.-H., Lee, J., Park, H., Zhang, M., Lee, Y.-I., Choa, Y.-H. and Myung, N.V. (2013) Template-Free Synthesis of Vertically Oriented Tellurium Nanowires via a Galvanic Displacement Reaction. Electrochimica Acta, 111, 200-205. https://doi.org/10.1016/j.electacta.2013.07.228
- 11. Li, W.-J., Yu, W.-L. and Yen, C.-Y. (2011) Pulsed Electrodeposition of Bi2Te3 and Bi2Te3/Te Nanowire Arrays from a DMSO Solution. Electrochimica Acta, 58, 510- 515. https://doi.org/10.1016/j.electacta.2011.09.075
- 12. Ma, Y., Ahlberg, E., Sun, Y., Iversen, B.B. and Palmqvist, A.E.C. (2011) Thermoelectric Properties of Thin Films of Bismuth Telluride Electrochemically Deposited on Stainless Steel Substrates. Electrochimica Acta, 56, 4216-4223. https://doi.org/10.1016/j.electacta.2011.01.093
- 13. Wang, L.P., Lin, Y.M., Zeng, Z.X., Liu, W.M., Xue, Q.J., Hu, L.T. and Zhang, J.Y. (2007) Electrochemical Corrosion Behavior of Nanocrystalline Co Coatings Explained by Higher Grain Boundary Density. Electrochimica Acta, 52, 4342-4350. https://doi.org/10.1016/j.electacta.2006.12.009
- 14. Ragavendran, K., Sherwood, D., Vasudevan, D. and Emmanuel, B. (2009) On the Observation of a Huge Lattice Contraction and Crystal Habit Modifications in LiMn2O4 Prepared by a Fuel Assisted Solution Combustion. Physica B: Condensed Matter, 404, 2166-2171. https://doi.org/10.1016/j.physb.2009.04.019





