Journal of Environmental Protection
Vol.07 No.04(2016), Article ID:64877,13 pages
10.4236/jep.2016.74048
Origanum majorana Extracts as Mild Steel Corrosion Green Inhibitors in Aqueous Chloride Medium
Hassen Challouf1,2, Nébil Souissi1, Mhamed Ben Messaouda3, Rym Abidi2, Adel Madani4
1Tunis El Manar University, Institut Préparatoire aux Etudes d’Ingénieurs El Manar, Campus Universitaire El Manar, Tunis, Tunisia
2Carthage University, Faculté des Sciences de Bizerte, Laboratoire de Recherche “Application de la Chimie aux Ressources et Substances Naturelleset à l’Environnement”, Jarzouna, Tunisia
3Carthage University, Institut Préparatoire aux Etudes Scientifiques & Techniques, Laboratoire de Recherche “Matériaux, Molécules et Applications”, La Marsa, Tunisia
4Carthage University, Faculté des Sciences de Bizerte, Laboratoire de Physique des Matériaux, Jarzouna, Tunisia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 18 January 2016; accepted 19 March 2016; published 23 March 2016
ABSTRACT
We optimized Origanum majorana (OM) extraction for mild steel corrosion inhibition in neutral 0.5 M chloride medium. The inhibition mechanism evolved in presence of the optimal extract was discussed when calculating the activation energy (Ea), the activation enthalpy (∆aH) as well as the activation entropy (∆aS). The OM extract molecules were investigated using the density functional theory (DFT) at B3LYP/6-31G(d,p) basis set level. 1-methyl-4-propan-2-ylcyclohexa-1,3-diene alpha- terpinene was predicted exhibiting the most inhibition capabilities.
Keywords:
Origanum majorana, Corrosion, Inhibition, Mild Steel, NaCl

1. Introduction
In recent years, many alternative eco-friendly corrosion inhibitors have been developed. They range from rare earth elements [1] [2] , inorganic compounds [3] to plants extracts [4] - [12] . However, few investigations were focused on the effect of the extraction experimental conditions on materials corrosion inhibition.
There are environmental issues associated with the application of most inhibitors as some are toxic to the ecosystem. Plants extracts are eco-friendly and have been found to contain phytochemical constituents with similar characteristics to organic corrosion inhibitor; hence, their applicability as inhibitors has been reported. The use of wastes from plants as corrosion inhibitors can be another way of extending the beneficial use of these plants and so enhance municipal waste management.
Origanum majorana is a somewhat cold-sensitive perennial herb or undershrub with sweet pine and citrus flavors. In some Middle Eastern countries, marjoram is synonymous with oregano, and there the names sweet marjoram and knotted marjoram are used to distinguish it from other plants of the genus Origanum. It is also called pot marjoram, although this name is also used for other cultivated species of Origanum.
This research program aimed at the optimization of the extraction procedure of green inhibitors from OM plants for mild steel preservation in aqueous chloride medium. While the optimum extract found, the inhibition mechanism was tentatively investigated. Quantum chemical calculations were also performed in order to correlate the electronic structures of the OM extracts molecules to the inhibition efficiency.
2. Experimental
2.1. Extraction of the Green Inhibitors from OM
Origanum majorana leaves (OM) were cut separately into pieces which were then oven dried at 180˚C for 20 minutes. They were separately ground into powder and soaked in different containers containing solvent in order to obtain the extract by leaching. Each of the different extracts was filtered at the end of the extraction period and stored in a clean bottle.
2.2. Electrochemical Study
Working electrodes were elaborated from XC48 mild steel. A classical three-electrode cell was used for the electrochemical characterizations with a saturated calomel electrode as reference and a platinum wire as counter electrode. The measurements were performed using PGSTAT 30 potentiostat. GPES software was used for instrumentation control. All tests were repeated at least three times. Polarization measurements were performed from −1.200 V to −0.4 V at a sweep rate of 10 mV/s. The curves were taken after 2 hours of exposition in pure 0.5 M NaCl solution without and with plant extract addition.
2.3. Computational Method
Quantum calculations were performed using the Gaussian (version 03) program. Exchange and correlation calculations were investigated using the functional hybrid DFT B3LYP and the 6-31++G(d,p) orbital basis sets for all atoms.
3. Results and Discussion
3.1. Modeling of the Parameters for Green Inhibitors Extraction from OM
In order to model the experimental conditions of Oriaganum majorana green inhibitors extraction for mild steel corrosion inhibition, a chemiometric approach was used.
After preliminary experiments, four factors were choosing:
U1: first factor representing the plant mass;
U2: second factor representing the solvent volume;
U3: third factor representing the extraction duration;
U4: fourth factor representing the solvent.
The experimental field is given in Table 1.
Table 1. Experimental field.
To compare the effects of the different factors in the experimental field considered, Ui were transformed into coded variables Xi as in previous works [13] [14] .
A mathematical approach was used to correlate the variables to the responses as:
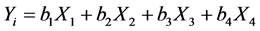 (1)
(1)
where:
bi: the estimation of the main effects of the factor i. Calculation of coefficients was carried out through the least squares method [13] [14] .
Yi: the experimental responses representing the corrosion potential (Ecorr), the anodic slope (βa), the cathodic slope (βc) and the corrosion current (Icorr) extracted. We also modelled the facor (B), the polarisation resistance (Rp) and the percentage of inhibition efficiency (%IE) calculated as follows:
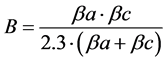 (2)
(2)
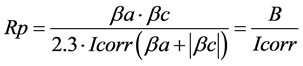 (3)
(3)
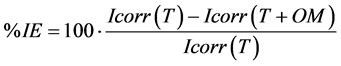 (4)
(4)
The eight experiments of the experimental design are reported in Table 2.
The polarization curves for mild steel immersed for two hours in NaCl 0.5 mol∙L−1 at 303 K in absence and in presence of OM extracts are given in Figure 1. These curves were plotted in the potentiostatic mode from −0.4 V to −1.2V at a scan rate of 10 mV∙s−1.
It was shown that independently on the extract experimental conditions; the anodic part of the polarization curves indicated material active dissolution. The cathodic part of the curves can be divided into two regions. The oxygen reduction reaction was limited by diffusion at intermediate potentials and possibly by an activation- controlled rate-determining step [15] . At high negative potentials solvent reduction was evolved.
Extrapolation of linear lines to the corrosion potential Ecorr allowed the determination of both βa and βc and the intercept gave the corrosion current Icorr. We calculated also B, Rp and % IE values using the above mentioned relationships.
The experimental results are reported in Table 3.
The responses models coefficients calculated as in [13] [14] and references therein are gathered in Table 4.
In order to evaluate the experimental factors effects, graphical Pareto analysis was performed as in [13] (Figure 2).
Table 2. Experimental matrix-Experimental design.
Table 3. Experimental results.
Table 4. Mathematical model coefficients.
Figure 1. Polarization curves of mild steel immersed at 303 K during two hours in NaCl 0.5 mol∙L−1 without and with addition of OM extract-Potentiostatic mode from −1.2 V to −0.4 V at a scan rate of 10 mV∙s−1.
It was observed that:
・ b1 alone could explain 96.7% of Ecorr variation;
・ about 90% of βa evolution was attributed to b3 and b2, the most important factor was the extraction duration (P3 = 59.4);
・ b1 and b3 were assumed responsible of 98% of βc variation as P1 was about 52% then the plant mass was the major factor;
・ factor B variation of was due to all factors and the plant mass was the most important factor (P1 = 33.6);
・ b3, b2, b1 could explain 94.6% of corrosion current evolution Icorr, the most important factor was the extraction duration (P3 = 66%);
・ b1, b3 and b4 could be responsible of 94% of Rp evolution, the most important factor was the plant mass
Figure 2. Pareto chart for the modelling of OM extraction parameters for mild steel.
(P1 = 48);
・ 92.1% of %IE variation could be attributed to b3, b2 and b4; the main factor was extraction duration (P3 = 63.7).
It could be retained that U2, U3 and U4 do not affect Ecorr. Furthermore, the anodic interfacial behavior is not conditioned by the plant mass and the solvent whereas the cathodic behavior was independent on the solvent nature and volume.
It was confirmed that B deviation depended on the experimental conditions as cited for copper corrosion in neutral chloride media [16] .
Similarities were evidenced for Icorr and %IE as for both responses up to 80% of their variations were attributed to the extraction duration and the solvent volume. However, the solvent was not relevant for the first response whereas the plant mass was not influent for the second.
For determining the optimal extraction conditions, %IE was choosing as the criteria. As b2 < 0, b3 > 0 and b4 < 0, U2, U3 and U4 must be fixed at −1, +1 and −1 respectively to maximize the inhibition efficiency.
Hence, the 6th experience of the experimental design (Table 2) corresponds to the optimal conditions of green inhibitors extraction from OM for mild steel corrosion preservation.
For such extraction condition, %IE reached about 90%. Furthermore, both βa and βc were affected when the optimal extract added to the corrosive media. The optimal extract acted as mixed type inhibitor.
3.2. Effect of Temperature
The effect of temperature on the corrosion phenomenon of mild steel in free and inhibited with the optimal extract of OM solutions of 0.5 M NaCl was studied in the temperature range 303 K - 318 K. The curves obtained are given in Figure 3.
The analysis of these figures revealed that raising the temperature increased both anodic and cathodic current densities, and consequently the corrosion current of XC48 steel increased.
When studying the Tafel region of the polarisation curves corrosion parameters were evaluated (Table 5).
The obtained corrosion parameters are given in Table 5.
Analyse of the results in Table 5 indicates that in the presence of OM molecules, the Icorr of XC48 steel decreases at any given temperature increases compared to the uninhibited solution, due to the increase of the surface coverage degree [17] .
Inhibition efficiencies of the inhibitor at different temperatures are shown by Figure 4. It is observed from Figure 3 that, with an increase in the temperature, the inhibition efficiency decreases. The inhibitor shows maximum inhibition at 303K. This gave a clue that the mechanism of adsorption of the inhibitor may be due to physisorption, because the physisorption is due to weak van der Waal’s forces, which disappear at elevated temperatures [18] .
The activation energy of corrosion process in free and inhibited solutions could be calculated using the equation:
Figure 3. Polarization curves of mild steel immersed in temperature range 303 K - 318 K during two hours in NaCl 0.5 mol∙L−1 without and with addition of OM optimal extract-Potentiostatic mode from −1.2 V to −0.4 V at a scan rate of 10 mV∙s−1.
Figure 4. Variation of inhibition efficiency with different temperatures.
Table 5. Electrochemical parameters for mild steel steel immersed in temperature range 303 K - 318 K during two hours in NaCl 0.5 mol∙L−1 without and with addition of OM extract.
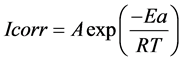 (5)
(5)
where Ea is the activation energy, A the frequency factor, T the absolute temperature, R the gas constant and Icorr is the corrosion current.
Figure 5 represents the relationship between ln(Icorr) and 103/T. Straight-lines are obtained in free chloride and in the presence of OM optimal extract. The corresponding activation energies were calculated from the slopes of Arrhenius plots. 20.7 kJ∙mol−1 and 64.7 kJ∙mol−1 were respectively the activation energy without and with the inhibitor.
Activation energy, Ea values are higher for inhibited solution than the uninhibited one, indicating a strong
Figure 5. Arrhenius diagram for mild steel immersed in NaCl 0.5 mol∙L−1 without and with addition of OM optimal extract.
inhibitive action of the additives by increasing energy barrier for the corrosion process, emphasizing the electrostatic character of the inhibitor’s adsorption on the mild steel surface (physisorption) [19] .
Another thermodynamic parameter which further describes the adsorption mechanism operative in the corrosion inhibition process is the heat of adsorption, Qads. In this regard, Qads was evaluated from the variation of surface coverage with reciprocal of temperature from the kinetic/thermodynamic model [19] , through the relation:

where A is a constant, C is the inhibitor concentration, θ is the occupied, and (1 − θ) is the vacant site not occupied by the inhibitor. The plot of 
The activation enthalpy (ΔaH) and the entropy of activation (ΔaS) for the corrosion of mild steel in 0.5 M NaCl in the absence and in the presence of OM extract were obtained by applying the alternative formulation of Arrhenius equation [17] :

where h is the Plank’s constant and N is the Avogadro’s number.
Figure 7 shows a plot of ln(Icorr/T) versus 103/T. Straight lines were$ obtained with a slope of (−ΔaH/R) and an intercept of (lnR/Nh + ΔaS/R) from which the values of ΔaH and ΔaS were calculated (Table 6).
The positive values of ΔaH in the absence and the presence of OM extract reflect the endothermic nature of the mild steel dissolution process [17] and it indicates that the dissolution of steel is difficult [19] .
The values of activation entropy (ΔaS) are higher for inhibited solutions than that for the uninhibited solution. The shift towards positive values of entropies (ΔaS) imply that the activated complex in the rate determining step represents dissociation rather than association, meaning that disordering increases on going from reactants to the activated complex [20] .
Figure 6. Plot of log (θ/1 − θ) vs 1/T for mild steel in 0.5 M NaCl containing OM extract.
Figure 7. Variation of Ln(Icorr/T) versus 103/T on XC48 steel in 0.5 M NaCl without and with addition of OM extract.
Table 6. Thermodynamic parameters of the XC48 steel in 0.5 M NaCl without and with addition of OM extract.
3.3. Quantum Chemical Studies
The main objective of this work was to investigate computationally the OM extract, using DFT at B3LYP/6-31G level of theory. Theoretical approaches provide useful means of analyzing the interaction between the inhibitor molecule and the metal. Furthermore, the quantum level calculation of molecules can explain also the mechanism of corrosion inhibition [21] .
The majority molecules of OM extracts denoted hereafter MEANE, ISEOL, ISANE, CILOL, MEONE1 and MEONE2 as shown in Figure 8.
The quantum chemical parameters correlated to the inhibition efficiency are εHOMO (the highest occupied molecular orbital energy), εLUMO (the lowest unoccupied molecular orbital energy), the energy gap (Δε), dipole moment (μ) (Figure 9).
The energy of the highest occupied molecular orbital (εHOMO) measured the tendency towards the donation of electron by a molecule [22] . Therefore, higher values of εHOMO indicated better tendency towards the donation of electron, enhancing the adsorption of the inhibitor on mild steel and therefore better inhibition efficiency. εLUMO indicated the ability of the molecule to accept electrons. The binding ability of the inhibitor to the metal surface increased with increasing of the HOMO and decreasing of the LUMO energy values.
The results of our present work show that MEANE has good protective effect for mild steel and has high εHOMO and small εLUMO values.
In the other hand, the results show that CILOL molecule have less HOMO energies and therefore are less electron donor than MEANE. Hence, in the interaction with the metal surface, CILOL are most likely to interact with the metal surface through physisorption mechanism while MEANE molecule would preferentially interact with the metal surface through chemisorption mechanism [23] .
Another point to be considered in energy level is the gap between the HOMO and LUMO energies for the inhibitor. As Δε decreased, the reactivity of the molecule increased leading to increase of the %IE. Lower values of the energy difference will render good inhibition efficiency, because the energy to remove an electron from the last occupied orbital will be low [24] . The results as indicated in Figure 9(a) shows that MEANE has the lowest energy gap, this means that the molecule could have better performance as corrosion inhibitor.
The low Δε value (4.814) again indicates higher reactivity of the MEANE with respect to adsorption on the metal surface. It is possible to suggest that the MEANE molecule plays an important role in the chemisorption of the OM extract on the metal surface. This observation implies that the inhibition process did proceed through electron transfer or acceptance in the interaction between the inhibitor and steel surface.
Figure 8. The majority molecules of OM extract.
Figure 9. µ, εHOMO, εLUMO, Δε for the majority molecules of OM.
As for the dipole moment µ (Figure 9(b)), low values of dipole moment favour inhibitor molecules accumu- lation on the surface thus increasing the inhibition effectiveness [25] . In our study the values 0.045 (Debye) of ISANE and 0.546 (Debye) of MEANE enumerates its better inhibition efficiency.
Some HSAB (Hard and Soft Acids and Bases) parameters such as electronegativity (χ), chemical hardness (η) can be expressed as follows in terms of εHOMO, εLUMO the highest occupied molecular orbital energy, and the lowest unoccupied molecular orbital energy, respectively: [26] [27] as:


The calculated values for the three parameters are reported in Figure 10.
The Figure 10(a) shows the order of electronegativity as MEANE < ISANE < MEONE2 < MEONE1 < ISEOL < CILOL. Hence an increase in the difference of electronegativity between the metal and the inhibitor is observed in the order. According to Sanderson’s electronegativity equalization principle [28] , CILOL with a high electronegativity and low difference of electronegativity quickly reaches equalization and hence low reactivity is expected which in turn indicates low inhibition efficiency. In contrary, MEANE was expected exhibiting the most inhibiting capabilities among the OM extract molecules.
Hardness (Figure 10(b)) is important properties to measure the molecular stability and reactivity. A hard molecule has a large energy gap and a soft molecule has a small energy gap. In our present study MEANE with low hardness value 2.407 (eV) compared with other compound have a low energy gap. Normally, the inhibitor with the least value of global hardness (hence the highest value of global softness) is expected to have the highest inhibition efficiency.
Electronegativity, hardness and softness have proved to be very useful quantities in the chemical reactivity theory. When two systems, Fe and inhibitor, are brought together, electrons will flow rom lower χ(inhibitor) to higher χ(metal), until the chemical potentials become equal.
For the metal surface, the work-function (Φ) is taken as its electronegativity, whereas the chemical hardness is neglected because η of bulk metals is related to the inverse of their density of states at the Fermi level-an exceedingly small number [29] . The number of transferred electrons (ΔN) was also calculated by using the equation below:

where:
χmetal: metal electronegativity;
χmol: molecule electronegativity;
Φ: work-function;
ηmetal: metal chemical hardness;
ηmol: molecule chemical hardness.
If ΔN < 3.6 [30] , the inhibition efficiency increases by increasing electron-donating ability of these inhibitors to donate electrons to the metal surface. In this study (Figure 11), the highest fraction of electrons transferred is
Figure 10. χ and η evolution for the majority molecules of OM.
Figure 11. Fraction of electrons transferred ΔN evolutions for the majority molecules of OM extract.
associated with the best inhibitor (MEANE) while the least fraction is associated with the inhibitor that has the least inhibition efficiency (CILOL).
4. Conclusion
From the present study, it was found that the extract of Origanum majorana leaves can be used as an inhibitor for mild steel corrosion. The optimization of the extraction parameters using chemiometric approach method gave the optimal extract of OM. The inhibition efficiency measured through polarisation curves can reach about 90%. The presence of the extract increased the activation energy which can be attributed to the physical adsorption of the OM molecules on the surface of steel. Thermodynamic adsorption parameters show that OM is adsorbed on steel surface by an exothermic process. The positive value of ΔaS suggests that an increase in randomness occurred on going from reactants to the activated complex. The parameters like hardness (η), dipole moment (μ), electronegativity (χ) and the fraction of electron transferred (ΔN) confirm that the MEANE molecule could be responsible for the inhibition action.
Cite this paper
HassenChallouf,NébilSouissi,Mhamed BenMessaouda,RymAbidi,AdelMadani, (2016) Origanum majorana Extracts as Mild Steel Corrosion Green Inhibitors in Aqueous Chloride Medium. Journal of Environmental Protection,07,532-544. doi: 10.4236/jep.2016.74048
References
- 1. Blin, F., Koutsoukos, P., Klepetsianis, P. and Forsyth M. (2007) The Corrosion Inhibition Mechanism of New Rare Earth Cinnamate Compounds—Electrochemical Studies. Electrochimica Acta, 52, 6212-6220.http://dx.doi.org/10.1016/j.electacta.2007.04.001
- 2. Yasakau, K.A., Zheludkevich, M.L., Lamaka, S.V. and Ferreira, M.G.S. (2006) Mechanism of Corrosion Inhibition of AA2024 by Rare-Earth Compounds. Journal of Physical Chemistry B, 110, 5515-5528.http://dx.doi.org/10.1021/jp0560664
- 3. Amin, M.A., Hassan, H.H. and Abd El Rehim, S.S. (2008) On the Role of Ions in Passivity Breakdown of Zn in Deaerated Neutral Sodium Nitrite Solutions and the Effect of Some Inorganic Inhibitors Potentiodynamic Polarization, Cyclic Voltammetry, SEM and EDX Studies. Electrochimica Acta, 53, 2600-2609.http://dx.doi.org/10.1016/j.electacta.2007.10.034
- 4. Abiola, O.K. and James, A.O. (2010) The Effects of Aloe vera Extract on Corrosion and Kinetics of Corrosion Process of Zinc in HCl Solution. Corrosion Science, 52, 661-664. http://dx.doi.org/10.1016/j.corsci.2009.10.026
- 5. Quraishi, M.A., Singh, A., Singh, V.K., Yadav, D.K. and Singh, A.K. (2010) Green Approach to Corrosion Inhibition of Mild Steel in Hydrochloric Acid and Sulphuric Acid Solutions by the Extract of Murraya koenigii Leaves. Materials Chemistry and Physics, 122, 114-122. http://dx.doi.org/10.1016/j.matchemphys.2010.02.066
- 6. Hamed, E. (2010) Studies of the Corrosion Inhibition of Copper in Na2SO4 Solution Using Polarization and Electrochemical Impedance Spectroscopy. Materials Chemistry and Physics, 121, 70-76.http://dx.doi.org/10.1016/j.matchemphys.2009.12.044
- 7. Lebrini, M., Robert, F., Lecante, A. and Roos, C. (2011) Corrosion Inhibition of C38 Steel in 1 M Hydrochloric Acid Medium by Alkaloids Extract from Oxandra asbeckii Plant. Corrosion Science, 53, 687-695.http://dx.doi.org/10.1016/j.corsci.2010.10.006
- 8. Hussin, M.H. and Kassim, M.J. (2011) The Corrosion Inhibition and Adsorption Behavior of Uncaria gambir Extract on Mild Steel in 1 M HCl. Materials Chemistry and Physics, 125, 461-468.http://dx.doi.org/10.1016/j.matchemphys.2010.10.032
- 9. Al-Turkustani, A.M., Arab, S.T. and Al-Qarni, L.S.S. (2011) Medicago sative Plant as Safe Inhibitor on the Corrosion of Steel in 2.0 M H2SO4. Journal of Saudi Chemical Society, 15, 73-82. http://dx.doi.org/10.1016/j.jscs.2010.10.008
- 10. Deng, S. and Li, X. (2012) Inhibition by Ginkgo Leaves Extract of the Corrosion of Steel in HCl and H2SO4 Solutions. Corrosion Science, 55, 407-415. http://dx.doi.org/10.1016/j.corsci.2011.11.005
- 11. Behpour, M., Ghoreishi, S.M., Khayatkashani, M. and Soltani, N. (2012) Green Approach to Corrosion Inhibition of Mild Steel in Two Acidic Solutions by the Extract of Punica granatum Peel and Main Constituents. Materials Chemistry and Physics, 131, 621-633. http://dx.doi.org/10.1016/j.matchemphys.2011.10.027
- 12. Al-Otaibi, M.S, Al-Mayouf, A.M., Khan, A., Mousa, A.A., Al-Mazroa, S.A. and Alkhathlan, H.Z. (2014) Corrosion Inhibitory Action of Some Plant Extracts on the Corrosion of Mild Steel in Acidic Media. Arabian Journal of Chemistry, 7, 340-346. http://dx.doi.org/10.1016/j.arabjc.2012.01.015
- 13. Souissi, N. and Triki, E. (2007) A Chemiometric Approach for Phosphate Inhibition of Copper Corrosion in Aqueous Media. Journal of Materials Science, 42, 3259-3265. http://dx.doi.org/10.1007/s10853-006-0809-x
- 14. Souissi, N. and Triki, E. (2008) Modelling of Phosphate Inhibition of Copper Corrosion in Aqueous Chloride and Sulphate Media. Corrosion Science, 50, 231-241. http://dx.doi.org/10.1016/j.corsci.2007.06.022
- 15. Abdel-Gaber, A.M., Khamis, E. and Hefnawy, A. (2011) Utilizing Arghel Extract as Corrosion Inhibitor for Reinforced Steel in Concrete. Materials and Corrosion, 62, 1159-1162. http://dx.doi.org/10.1002/maco.201005653
- 16. Kear, G., Barker, B.D. and Walsh, F.C. (2004) Electrochemical Corrosion of Unalloyed Copper in Chloride Media—A Critical Review. Corrosion Science, 46, 109-135. http://dx.doi.org/10.1016/S0010-938X(02)00257-3
- 17. Labjar, N., Bentiss, F., Lebrini, M., Jama, C. and El hajjaji, S. (2011) Study of Temperature Effect on the Corrosion Inhibition of C38 Carbon Steel Using Amino Tris(Methylenephosphonic) Acid in Hydrochloric Acid Solution. International Journal of Corrosion, 2011, Article ID: 548528. http://dx.doi.org/10.1155/2011/548528
- 18. Mayakrishnan, G., Pitchai, S., Raman, K., Ramani Vincent, A. and Nagarajan, S. (2011) Inhibitive Action of Clematis gouriana Extract on the Corrosion of Mild Steel in Acidic Medium. Ionics, 17, 843-852. http://dx.doi.org/10.1007/s11581-011-0584-9
- 19. Eduok, U.M., Umoren, S.A. and Udoh, A.P. (2012) Synergistic Inhibition Effects between Leaves and Stem Extracts of Sida acuta and Iodide Ion for Mild Steel Corrosion in 1 M H2SO4 Solutions. Arabian Journal of Chemistry, 5, 325- 337. http://dx.doi.org/10.1016/j.arabjc.2010.09.006
- 20. Singh, A., Singh, V.K. and Quraishi, M.A. (2010) Effect of Fruit Extracts of Some Environmentally Benign Green Corrosion Inhibitors on Corrosion of Mild Steel in Hydrochloric Acid Solution. Journal of Materials and Environmental Science, 1, 162-147.
- 21. Nofrizal, S., Rahim, A.A., Saad, B., Bothi Raja, P., Shah, A.M. and Yahya, S. (2012) Elucidation of the Corrosion Inhibition of Mild Steel in 1 M HCl by Catechin Monomers from Commercial Green Tea Extracts. Metallurgical and Materials Transactions, 43, 1382-1393. http://dx.doi.org/10.1007/s11661-011-1030-3
- 22. El Ashry, H.E., El Nemr, A., Esawy, S.A. and Ragab, S. (2006) Corrosion Inhibitors: Part II: Quantum Chemical Studies on the Corrosion Inhibitions of Steel in Acidic Medium by Some Triazole, Oxadiazole and Thiadiazole Derivatives. Electrochimica Acta, 51, 3957-3968. http://dx.doi.org/10.1016/j.electacta.2005.11.010
- 23. Kabanda, M.M., Murulana, L.C., Ozcan, M., Karadag, F., Dehri, I., Obot, I.B. and Ebenso, E.E. (2012) Quantum Chemical Studies on the Corrosion Inhibition of Mild Steel by Some Triazoles and Benzimidazole Derivatives in Acidic Medium. International Journal of Electrochemical Science, 7, 5035-5056.
- 24. Obot, I.B., Obi-Egbedi, N.O. and Umoren, S.A. (2010) Adsorption Characteristics and Corrosion Inhibitive Properties of Clotrimazole for Aluminium Corrosion in Hydrochloric Acid. International Journal of Electrochemical Science, 4, 863-877.
- 25. Khalil, N. (2003) Quantum Chemical Approach of Corrosion Inhibition. Electrochimica Acta, 48, 2635-2640. http://dx.doi.org/10.1016/S0013-4686(03)00307-4
- 26. Parr, R.G. and Pearson, R.G. (1983) Absolute Hardness: Companion Parameter to Absolute Electronegativity. Journal of the American Chemical Society, 105, 7512-7516. http://dx.doi.org/10.1021/ja00364a005
- 27. Pearson, R.G. (1988) Absolute Electronegativity and Hardness: Application to Inorganic Chemistry. Inorganic Chemistry, 27, 734-740. http://dx.doi.org/10.1021/ic00277a030
- 28. Geerlings, P. and De Proft, F. (2002) Chemical Reactivity as Described by Quantum Chemical Methods. International Journal of Molecular Sciences, 3, 276-309. http://dx.doi.org/10.3390/i3040276
- 29. Yang, W. and Parr, R.G. (1985) Hardness, Softness and the Fukui Function in the Electronic Theory of Metals and Catalysis. Proceedings of the National Academy of Sciences of the United States of America, 82, 6723-6726. http://dx.doi.org/10.1073/pnas.82.20.6723
- 30. Lukovits, I., Kalman, E. and Zucchi, F. (2011) Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion, 57, 3-8. http://dx.doi.org/10.5006/1.3290328













