American Journal of Plant Sciences
Vol.06 No.04(2015), Article ID:54534,9 pages
10.4236/ajps.2015.64060
Photosynthetic Capacities and Productivity of Indoor Hydroponically Grown Brassica alboglabra Bailey under Different Light Sources
Jie He*, Lin Qin, Yunman Liu, Tsui Wei Choong
Natural Sciences and Science Education Academic Group, National Institute of Education, Nanyang Technological University, Singapore City, Singapore
Email: *jie.he@nie.edu.sg
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 January 2015; accepted 8 March 2015; published 11 March 2015
ABSTRACT
A major challenge for growing vegetables in an indoor vertical farming system will be supplying not only sufficient quantity but also quality of light. It has been reported that yield of crops is enhanced under appropriate combination of red and blue light compared with red light alone. This project aims to investigate the effects of different combinations of red and blue. Plants were cul- tured for a 12-h photoperiod at 210 µmol∙m−2∙s−1 photosynthetic photon flux density (PPFD) under different combinations of red (R) and blue (B) light-emitting diodes (LED). The R:B-LED ratios are: 1) 100:0 (0B); 2) 92:8 (8B); 3) 84:16 (16B) and; 4) 76:24 (24B). All combined RB-LEDs significantly increased light-saturated photosynthetic CO2 assimilation rate (Asat), stomatal conductance (gs sat) and productivity compared with those under 0B. Results suggested that 16B was the most suitable combination of LEDs to achieve the highest productivity for B. alboglabra. To further sub- stantiate these results, comparative studies were conducted under equal photoperiod and PPFD among 16B (RB-LED), white LED (RBW-LED) and high-pressure sodium (HPS) lamps. Shoot, root biomass, leaf number, leaf mass per area and Asat were higher in plants under HPS lamps and RB-LED, than under RBW-LED. However, gs sat was lower under RB-LED and RBW-LED, than under HPS lamps. Plants under RB-LED had higher electron transport rate and photochemical quenching but lower non-photochemical quenching than those under RBW-LED and HPS lamps. Thus, these results more conclusively affirmed that 16B was the most suitable light source to achieve the highest photosynthetic capacities. The findings of this study could also be used in vertical farming to achieve the highest productivity of vegetable crops such as B. alboglabra within the shortest growth cycle with reduced energy consumption.
Keywords:
Brassica alboglabra, LED Lighting, Photosynthesis, Productivity

1. Introduction
The maintenance of food security is increasingly challenging for modern cities, such as Singapore, where arable land is limited [1] . Urban indoor vertical farming system can offer a solution for the production of vegetable crops. The key determining factor for a successful indoor vertical farming system is the provision of uniform and effective lighting to the plants. For an indoor farming system, light is critical for vegetable production not only as the energy source for photosynthesis but also as a signal for morphogenesis [2] - [4] . Artificial lighting has been used to grow vegetables for almost 150 years [5] . Due to its higher electrical efficiencies and relatively broad emission spectrum that was suitable for a wide range of plant species [6] , high-pressure lamps such as high-pressure sodium (HPS) lamps, have been popular in growth chambers and greenhouses since 1950s. The 400-watt high HPS lamps have also been used by our team to grow vegetable for research. However, poor light uniformity, high thermal radiation and high energy consumption limit more extensive usage of HPS lamps in large scale of vegetable production. In early 1990s, after the testing of light-emitting diodes (LEDs) for plant growth in space [7] , much effort has been made to enhance not only the productivity but also quality of crops using LED lighting as artificial light sources. LEDs have numerous advantages such as fast switching, lower energy cost, higher durability, longer lifetime, lower thermal radiation, and narrower variation in specific wavelengths [8] for targeted crops. Although LEDs are more costly in comparison with conventional HPS lamps, the variation in spectrum and quantity of light could control costs more economically.
Quality of light is especially important for indoor vegetable production since the artificial light is the only light source for the vegetables. LEDs, unlike HPS lamps, allow combinations of spectra and intensity specifically tailored to plant production. It was reported that red and blue light from LED lighting promote dry matter production in pepper [9] , lettuce, spinach and radish crops when grown under appropriate combinations [10] .
Light spectrum influences plant productivity via: 1) direct effects on leaf photosynthesis [11] - [13] and 2) indirect effects on plant morphogenesis and developmental processes, which in turn interrelate with photosynthesis on plant and crop yield [7] [11] [14] [15] . In addition to providing the irradiance required for plant growth, LEDs can also be used to control the spectrum of light, allowing manipulation of plant photomorphogenetic and photosynthetic responses [7] . Understanding the effects of LED spectrum on leaf morphology and photosynthesis could allow for the selection of optimal conditions for vegetable production, at leaf level, at an early stage. This significantly adds to further optimizing plant production and saving energy.
Although the combination of RB-LED has great potential as a light source to drive photosynthesis, different vegetable crops may respond differently. The first part of this study was carried out by growing B. alboglabra plants under different combinations of RB-LEDs to select the optimal combination of RB-LED through the measurements of photosynthetic rate and productivity. The second purpose of this study was to determine the effect of LED lighting when comparing RB-LED and RBW-LED to HPS lamps via more detailed studies of shoot and root biomass, and leaf growth. Other than light-saturated photosynthetic rate (Asat) and stomatal conductance (gs and gs sat), photosynthetic light use efficiency was also studied to affirm the most suitable light source for the production of B. alboglabra.
2. Materials and Methods
2.1. Plant Cultivation under Different Combinations of RB-LEDs
Three days after germination, seedlings of B. alboglabra were inserted onto polyurethane cubes. The seedlings were then transferred to the growth room for 4 days for acclimatization before transplanting onto the hydroponic trays. All plants were cultured with a 12-h photoperiod of light and they were exposed to an equal photosynthetic photon flux density (PPFD) of 210 µmol∙m−2∙s−1 under each of the four R:B LED ratios: 1) 100:0 (0B); 2) 92:8 (8B); 3) 84:16 (16B) and; 4) 76:24 (24B). The spectra of four RB-LEDs are shown in Figure 1. The temperature and relative humidity in the growth room were 26˚C/24˚C (day/night) and 55%/75% (day/night),
Figure 1. Light spectral of the four different RB-LED light combinations. Spectral scans were recorded every 0.5 nm with a spectroradiometer (PS300, Apogee Instruments, USA).
respectively. They were supplied with full strength Netherlands Standard Composition [16] . Nutrient solution conductivity and pH 6.5 were maintained at 2 mS ± 0.2 and pH 6.5 ± 0.5, respectively.
2.2. Plant Cultivation under RB-LED, White LED (RBW-LED) and High-Pressure Sodium (HPS) Lamps
For comparative studies, in the 2nd part of the study, B. alboglabra plants were grown under HPS lamps and two different combinations of LEDs. The two combinations of LEDs are: R- (84%) and B- (16%) LED defined as RB-LED in this part of study, and white LED, named as RBW-LED. All plants were supplied with equal amount of PPFD of 210 µmol∙m−2∙s−1. The photoperiod and other growth conditions were also same as those stated in section 2.1. The spectra of HPS, RB-LED and RBW-LED are shown in Figure 2.
2.3. Measurements of Leaf Growth Parameter and Productivity
Leaf number was counted immediately after harvest before measuring shoot and root fresh weight (FW) that was recorded using a weighing balance (Sartorius, Fisher General Scientific Private Limited, Singapore). After recording the FW, total leaf areas were immediately measured by WinDIAS 3 Image Analysis System (Delta-T Device, Led, UK). After leaf area measurements, both shoot and root tissues were wrapped in aluminium foil and dried in an oven at 80˚C for 5 days. Samples were then weighed to determine dry weight (DW). Leaf mass per unit area (LMA) was calculated by dividing leaf dry mass over leaf area.
2.4. Measurement of Asat and gs sat
They were measured simultaneously with an open infrared gas analysis system with a 6 cm2 chamber (LI-6400, Biosciences, US), 3 hours after plants were exposed to different light sources. Readings were measured with a LED light source, which supplied a saturated PPFD of 1000 µmol∙m−2∙s−1. The light source emitted light in the wavelength range of 660 to 675 nm. Average ambient [CO2] in the chamber was 400 ± 5 µmol∙mol−1.
2.5. Measurement of Midday gs and Leaf Temperature
The gs and leaf temperature were measured using the leaf porometer chamber (SC-1, Decagon, US) with a fixed diffusion path to the leaf surface.
2.6. Measurement of Electron Transport Rate (ETR), Photochemical Quenching (qP) and Non-Photochemical Quenching (NPQ) of Chlorophyll Fluorescence
Leaves were harvested at 0090 h and were pre-darkened for 15 min prior to measurements. ETR, qP and NPQ were determined using the Imaging Pam Chl Fluorometer (Walz, Effeltrich, Germany) at 25˚C under different
Figure 2. Spectral distribution (300 - 1100 nm) of light from HPS lamps, RBW-LED and RB-LED. Spectral scans were recorded every 0.5 nm with a spectroradiometer (PS300, Apogee Instruments, USA).
PPFDs in the laboratory as described by He et al. [17] . By using the PAM Chl Fluorometer, images of fluorescence emission were digitized within the camera and via a Firewire interface (400 megabits/s) (Firewire-1394, Austin, TX, USA) to a personal computer for storage and analysis. Low frequency (about 1 Hz) of measuring light pulses was applied to obtain the initial chlorophyll fluorescence, Fo images in quasi-dark state. During actinic light illumination and saturation pulses, the frequency of measuring light pulses and image capture was automatically increased to about 10 Hz. The Imaging-PAM continuously measured the current fluorescence (Ft). In the absence of actinic illumination and upon application of a saturating, the dark-level fluorescence yield (Fo) and the maximum fluorescence yield (Fm) were determined respectively. After measurements of Fo and Fm, rapid light curve measurements in the presence of actinic light [18] were obtained through the application of a series of 10-s light exposures with increasing irradiance from 1 to 1585 µmol∙photons∙m−2∙s−1. In the presence of actinic light, the current fluorescence yield (Ft), and the maximum fluorescence (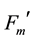 ) at the steady state, were
) at the steady state, were
determined, from which the effective PSII quantum yield,
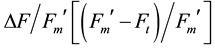 and ETR
and ETR
(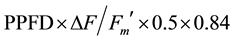 ) [19] could be calculated. The use of two photons is necessary to transport one electron (factor 0.5). Correction factor 0.84 takes into account that only a fraction of incident light is really absorbed by photosynthesis [19] . NPQ was defined as:
) [19] could be calculated. The use of two photons is necessary to transport one electron (factor 0.5). Correction factor 0.84 takes into account that only a fraction of incident light is really absorbed by photosynthesis [19] . NPQ was defined as: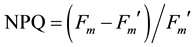 .
.
2.7. Statistical Analysis
One-way ANOVA was used to test for significant differences among different light treatments. Tukey’s multiple comparison tests were used to discriminate the means (MINITAB, Inc., Release 15, 2007).
3. Results
3.1. Productivity, Photosynthetic Gas Exchange of B. alboglabra Grown under Different Combinations of RB-LEDs
All combined RB-LEDs (8B, 16B and 24B) enhanced leaf number, leaf area (Table 1), leaf mass per unit area (LMA) and shoot and root FW and DW compared with those grown solely under R-LED (0B) (Table 1). Among the different combined RB-LED, 16B treatment resulted in the highest values of these parameters followed by those plants exposed to 24B and 8B conditions while plants grown under 0B conditions had the lowest values. Plants under all combined RB-LEDs had significantly higher average Asat and gs sat than those under 0B condition. Among the different combined RB-LEDs, plants grown under 16B for 18 days had the highest Asat although there was no significant difference in gs sat between 16B and 24B (Table 1).
3.2. Productivity and Photosynthetic Gas Exchange of B. alboglabra Grown under RB-LED, RBW-LED and HPS Lamps
There were no significant differences in shoot and root FW and DW between plants grown under HPS lamps
Table 1. Productivity and photosynthetic gas exchange of B. alboglabra grown under different RB-LEDs. Parameters of productivity were measured after harvest (21 days after transplanting) whereas Asat and gs sat were determine 3 days before harvest. Means with different letters are statistically different (P < 0.05; n = 7) as determined by Tukey’s multiple comparison test.
and RB-LED. However, these parameters were significantly higher in plants grown under HPS lamps and RB-LED than in plants exposed to RBW-LED (Figures 3(a)-(d)). Compared with plants grown under RBW- LED, plants grown under HPS lamps and RB-LED also had great leaf number (Figure 3(e)), bigger leaf area and higher LMA (Figure 3(f)).
Plants grown under HPS lamps and RB-LED had higher Asat compared to those grown under RBW-LED (Figure 4(a)). However, gs sat was much lower in plants grown under RBW-LED and RB-LED than under HPS lamps (Figure 4(b)). It is interesting to note that all plants had similar Ci (Figure 4(c)). Lower stomatal conductance in plants grown under RBW-LED and RB-LED was further confirmed by measuring midday gs of leaves using a porometer (Figure 5(a)). The higher midday gs in plants grown under HPS lamps was correlated with their higher leaf temperature (Tleaf) (Figure 5(b)).
3.3. Photosynthetic Light Utilization of B. alboglabra Grown under RB-LED, RBW-LED and HPS Lamps
The measurements were carried out after B. alboglabra plants grown under different light sources for 25 days. For all plants, ETR increased with increasing PPFD from 25 to 965 µmol∙m−2∙s−1. They were decreased with further increasing PPFD when measured under PPFDs > 965 µmol∙m−2∙s−1 (Figure 6(a)). Although the light response curves were similar among the different plants, at higher PPFDs from 605 µmol∙m−2∙s−1 onwards, the ETR values for plants grown under RB-LED were significantly higher than those plants grown under RBW- LED and HPS lamps. However, there were no significant differences in ETR between plants grown under RBW-LED and HPS lamps (Figure 6(a)). qP decreased with increasing PPFD for all plants (Figure 6(b)). No differences in qP values were observed between plants grown under RBW-LED and HPS lamps at any given PPFD. However, similar to those ETR, qP values were significantly higher in plants grown under RB-LED than those under RBW-LED and HPS lamps (Figure 6(b)) at higher PPFDs from 605 µmol∙m−2∙s−1 onwards. These results show that plants grown under RB-LED had a higher light utilization compared with those of plants under RBW-LED and HPS lamps. For heat dissipation measured as NPQ, all plants had similar treads. That was, values of NPQ increased gradually with increasing PPFDs from 25 to 1585 µmol∙m−2∙s−1 (Figure 6(c)). However, NPQ values were lower in plants grown under RB-LED compared with those grown under RBW-LED and HPS lamps from PPFD of 605 µmol∙m−2∙s−1 onwards (Figure 6(c)).
4. Discussion
Bula et al. (1992) [7] first reported that the growth of lettuce plants under red LEDs supplemented with blue fluorescent lamps was equivalent to that under cool-white fluorescent plus incandescent lamps. It was observed that lettuce and other dicotyledonous plants developed excessive hypocotyl and stem elongation, leaf extension,
Figure 3. FW and DW of shoot ((a), (b)) and root ((c), (d)), leaf number and LMA ((e), (f)) of plants grown under different light sources at harvest (25 days after treatments). Vertical bars represent the standard errors. Means with different letters are statistically different (P < 0.05; n = 6) as determined by Tukey’s multiple comparison test.
Figure 4. Asat (a), gs sat (b) and Ci (c) of B. alboglabra Bailey plants grown under different light sources for 25 days. Vertical bars represent the standard errors. Means with different letters are statistically different (P < 0.05; n = 7) as determined by Tukey’s multiple comparison test.
Figure 5. Midday gs (a) and Tleaf (b) of B. alboglabra Bailey plants grown under different light sources for 25 days. Vertical bars represent the standard errors. Means with different letters are statistically different (P < 0.05; n = 7) as determined by Tukey’s multiple comparison test.
and reduced chlorophyll when grown under only red LED. The abnormal morphological characteristics were eliminated when red LED was supplemented with blue light [7] . In the present study, B. alboglabra plants grown under combined RB-LED had higher total number of leaves and total leaf area when compared with red LED alone (Table 1). Plants grown under 16B had the highest values of both parameters. However, there were no significant differences in total chlorophyll and carotenoid content among the different RB-LED treatments (data not shown). Goins et al. [20] found that Arabidopsis leaf morphology was abnormal for plants grown
Figure 6. ETR (a), qP (b), and NPQ (c) of B. alboglabra Bailey plants grown under different light sources for 25 days. Vertical bars represent the standard errors. Means with different letters are statistically different (P < 0.05; n = 7) as determined by Tukey’s multiple comparison test.
under red alone with downward curling of leaf margins and spiral growth of the rosette, but inclusion of blue light at any level restored normal leaf morphology. However, abnormal leaf morphology was not observed under any RB-LED combination in the present study. Schuerger et al. [21] examined changes in leaf anatomy of pepper plants under different combinations of LEDs. It was found that under the same intensity of LED lighting, leaf thickness and number of chloroplasts per cell were dependent on the quantity of blue light. Plants grown under 20% of blue light had greatest leaf thickness and highest number of chloroplasts per cell. In the present study, thickest leaves, measured by LAM, was found in plants grown under 16B (16% of blue LED). Several other researchers reported that blue light played important roles in both morphogenesis and dry matter production [9] - [11] . Our study also found that B. alboglabra plants grown under 16B had the highest FW and DW compared with those grown under 0, 8 and 24B condition (Table 1). However, the absence of blue light did not cause any significant changes in total leaf area and total DW of white clover [22] .
It has been reported that red and blue light exerted effects on the photosynthetic apparatus [23] and thus, photosynthetic rate [4] [7] [10] - [12] [14] [15] . Much research has been carried out on the action spectrum of photosynthesis [24] . However, the effect of different RB-LED ratios on photosynthesis of specific species remains unclear [25] [26] . For instance, studies with red leaf lettuce, showed that the difference of 5% to 10% blue-LED changed total dry weight of the plants significantly. Further, changes of biomass accumulation and photosynthetic rate were also dependent on the ratios of combinations of RB-LED and amount of PPFD [26] . In wheat, photosynthetic rates were higher with increasing gs in leaves under red-LED combined with blue light [11] . It was suggested that the increase in photosynthetic rates might have resulted from increased gs. However, it was also reported that photosynthetic rates did not increase when stomatal opening was stimulated under red-LED supplemented with blue light [10] . In the study with lettuce plants, it was concluded that gs was responsive to spectral quality during growth and, in the short-term, but not coupled directly to dry matter accumulation [4] . Therefore, the effect of blue light on leaf photosynthesis and dry matter productivity remain unclear and the effects may be species-dependent. Under the same amount of PPFD, our present study found that B. alboglabra plants grown under combined RB-LED enhanced both Asat and gs sat compared with those under red-LED alone (Table 1). It was also found that 16B had the highest Asat and gs sat (Table 1). However, similar values of gs sat between plants grown under 16B and 24B were observed but that of lower Asat in plants grown under 24B was obtained. These results led us to postulate that higher amounts of blue-LED, for instance, 24B may cause some reversible damage on photosynthetic machinery reflected by healthy chlorophyll fluorescence
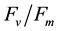 ratios of >0.8 (data not shown). However, due to extra energy expended on repairing processes, B. alboglabra plants under 24B had lower productivity than plants under 16B (Table 1). It was also thought photosynthetic rates changed with changes in pigment content and leaf composition [27] [28] . However, in the present study, although the values of Asat were different among different combinations of RB-LED, no significant differences in photosynthetic pigments were observed (data not shown). The above discussion demonstrates that the effect of red and blue-LED is species-dependent. On the other hand, the effects of different RB-LED ratios on plant growth and photosynthesis are dependent on light intensity [26] as well as quality, such as that of green [4] [29] [30] and far red light [9] [21] . These merit our future studies.
ratios of >0.8 (data not shown). However, due to extra energy expended on repairing processes, B. alboglabra plants under 24B had lower productivity than plants under 16B (Table 1). It was also thought photosynthetic rates changed with changes in pigment content and leaf composition [27] [28] . However, in the present study, although the values of Asat were different among different combinations of RB-LED, no significant differences in photosynthetic pigments were observed (data not shown). The above discussion demonstrates that the effect of red and blue-LED is species-dependent. On the other hand, the effects of different RB-LED ratios on plant growth and photosynthesis are dependent on light intensity [26] as well as quality, such as that of green [4] [29] [30] and far red light [9] [21] . These merit our future studies.
Owing to their acceptable spectrum and high energy efficiency, the traditional HPS lamps have become the artificial lighting used in the greenhouse horticulture since 1950s [5] . As mentioned earlier, the effects of LED lighting on plant growth and photosynthesis may also depend on other quality of light [4] [9] [21] [29] [30] . In the comparative studies, our results showed that plants grown under HPS lamps and RB-LED had similar higher shoot and root FW and DW (Figures 3(a)-(d)) compared with those plants grown under RBW-LED that included other qualities of light (Figure 2). Total leaf number and LMA also showed the similar responses (Figure 3(e), Figure 3(f)). Lower productivity of B. alboglabra plants under RBW-LED could be caused by higher blue with much lower red light in the spectrum (Figure 2). Similar to the results of B. alboglabra plants grown under 24B conditions discussed earlier, high ratio of blue light generated from RBW-LED (Figure 2) might result in some damage of photosynthetic machinery although the damage was revertible measured by chlorophyll fluorescence
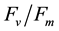 ratio (data not shown).
ratio (data not shown).
Based on their faster leaf growth and higher productivity as shown in Figure 3, higher Asat of B. alboglabra plants under both RB-LED and HPS lamps was expected, when compared with those under RBW-LED (Figure 4(a)). However, it was a surprise to note that gs sat was lower in plants under RB-LED and RBW-LED than under HPS lamps (Figure 4(b)). There were no significant differences in the intercellular CO2 concentration (Ci) among the plants under the different light treatments. These results indicated that neither gs sat nor Ci were major factors responsible for the differences in Asat among plants grown under different artificial lightings. Lower gs values of plants grown under RB-LED and RBW-LED were further confirmed during midday measured by a porometer (Figure 5(a)). The higher values of gs in plants grown under HPS lamps were correlated with their higher Tleaf (Figure 5(b)), indicating that differences in Tleaf can affect gs and Ci independently of Asat. Our understanding of photosynthetic responses to environmental factors is highly dependent on the relationship between Asat and Ci [31] . The values of Ci are normally the estimation of the CO2 mole fraction at the leaf surface [32] , based on the assumption that photosynthesis and transpiration are relatively uniform and gs is maximal over the leaf area. However, in the present study, the gs values of B. alboglabra plants grown indoor under different artificial lightings was much lower than our previous study with the same type of B. alboglabra plants grown in the greenhouse under naturals sunlight [33] . It was reported that stomatal opening was most responsive to light in the blue region of the spectrum than other wavelengths [11] [12] [34] and light quality also affected the stomatal development [12] [35] and other leaf traits such as LMA [12] . The present study also showed that plants grown under RB-LED had higher LMA (Table 1, Figure 3(f)). To understand the relationship among Asat, gs sat and Ci in vegetable crops grown under different artificial lightings, more research should be carried out to study the effects of different light sources on stomatal development and gs. Decreases in Asat, also partially described as a non-stomatal inhibition of photosynthesis, result from decreased photosynthetic utilization of radiant energy measured by chlorophyll fluorescence [33] [36] [37] .
Grown under red light only, there was dysfunction of the photosynthetic machinery in cucumber plants, in particular a loss of photochemical efficiency of PS II [12] . The main energy-consuming processes at PS II are photochemistry, heat dissipation and chlorophyll fluorescence [38] . Plants grown under RB-LED utilized more energy in photochemistry, as the higher ETR and qP (Figure 6(a)) have reflected, (Figure 6(b)) so less energy was dissipated as heat as reaffirmed by lower NPQ values (Figure 6(c)) at higher PPFD, than those grown under RBW-LED and HPS lamps. However, higher blue light generated from RBW-LED (Figure 2) seemed to have some negative impact on the photochemistry demonstrated by lower maximal ETR and qP but higher NPQ compared with RB-LED. It was reported that excessive blue light could cause photodamage of PS II [39] . As
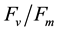 ratios did not decrease (data not shown), plants grown under RBW-LED condition might have spent additional energy to repair its PS II during the photoperiod.
ratios did not decrease (data not shown), plants grown under RBW-LED condition might have spent additional energy to repair its PS II during the photoperiod.
The balance between light intensity and temperature appeared to have a considerable influence on the utilization of light energy and heat dissipation. Plants grown under HPS lamps had lower ETR and qP but higher NPQ compared with those plants grown under RB-LED (Figure 6). At the same PPFD, a lower canopy temperature is measured under RB-LED than HPS lamps (Figure 5(b)), since RB-LED does not emit any near infrared radiation (Figure 2). Interaction between leaf temperature and light spectrum is poorly understood at the leaf levels of different vegetable crops [40] and this merits our further studies.
5. Conclusion
Based on the above discussion, RB-LED lighting could potentially play a major role in vegetable production since it produces less heat and uses less energy, than HPS lamps. The findings of this study could be used in vertical farming systems to achieve the highest productivity of vegetable crops such as B. alboglabra within the shortest growth cycle, with reduced energy consumption.
Acknowledgements
This project was supported by Singapore Millennium Foundation, Singapore.
Cite this paper
JieHe,LinQin,YunmanLiu,Tsui WeiChoong, (2015) Photosynthetic Capacities and Productivity of Indoor Hydroponically Grown Brassica alboglabra Bailey under Different Light Sources. American Journal of Plant Sciences,06,554-563. doi: 10.4236/ajps.2015.64060
References
- 1. He, J. and Lee, S.K. (2013) Impact of Climate Change on Food Security and Proposed Solutions for the Modern City. Acta Horticulturae, 1004, 41-52.
- 2. Berry, J.A. and Downton, W.J.S. (1982) Environmental Regulation of Photosynthesis. In: Govindjee, Ed., Photosynthesis (Vol. 2), Academic Press, New York, 294-306.
- 3. Sager, J.C. and Wheeler, R.M. (1992) Application of Sunlight and Lamps for Plant Irradiance in Space Bases. Advanced Space Research, 12, 133-140.
http://dx.doi.org/10.1016/0273-1177(92)90019-T - 4. Kim, H.H., Wheeler, R.M. and Sager, J.C. (2004) A Comparison of Growth and Photosynthetic Characteristics of Lettuce Grown under Red and Blue Light-Emitting Diodes (LEDs) with and without Supplemental Green LEDs. Acta Horticulturae, 659, 467-475.
- 5. Pfeiffer, N.E. (1926) Microchemical and Morphological Studies of Effect of Light on Plants. Botanical Gazette, 81, 173-195.
http://dx.doi.org/10.1086/333584 - 6. Cathey, H.M. and Campbell, L.E. (1980) Light and Lighting Systems for Horticultural Plants. Horticultural Reviews, 2, 491-537.
- 7. Bula, R.J., Tibbitts, T.W., Morrow, R.C. and Dinauer, W.R. (1992) Commercial Involvement in the Development of Space-Based Plant Growing Technology. Advanced Space Research, 12, 5-10.
http://dx.doi.org/10.1016/0273-1177(92)90002-F - 8. Massa, G.D., Kim, H.-H., Wheeler, R.M. and Mitchell, C.A. (2008) Plant Productivity in Response to LED Lighting. HortScience, 431, 1951-1956.
- 9. Brown, C.S., Schuerger, A.C. and Sager, J.C. (1995) Growth and Photomorphogenesis of Pepper Plants under Red Light-Emitting Diodes with Supplemental Blue or Far-Red Lighting. Journal of the American Society for Horticultural Science, 120, 808-813.
- 10. Yorio, N.D., Goins, G.D., Kagie, H.R., Wheeler, R.M. and Sager, J.C. (2001) Improving Spinach, Radish and Lettuce Growth under Red Light-Emitting Diodes (LEDs) with Blue Light Supplementation. HortScience, 36, 380-383.
- 11. Goins, G.D., Yorio, N.C., Sanwo, M.M. and Brown, C.S. (1997) Photomorphogenesis, Photosynthesis, and Seed Yield of Wheat Plants Grown under Red Light-Emitting Diodes (LEDs) with and without Supplemental Blue Lighting. Journal of Experimental Botany, 48, 1407-1413.
http://dx.doi.org/10.1093/jxb/48.7.1407 - 12. Hogewoning, S., Trouworst, G., Maljaars, H., Poorter, H., van Leperen, W. and Harbinson, J. (2010) Blue Light Dose-Response of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis sativus Grown under Different Combinations of Red and Blue Light. Journal of Experimental Botany, 61, 3107-3117.
http://dx.doi.org/10.1093/jxb/erq132 - 13. Trouwborst, G., Oosterkamp, J., Hogewoning, S.W., Harbinson, J. and van Ieperen, W. (2010) The Responses of Light Interception, Photosynthesis and Fruit Yield of Cucumber to LED-Lighting within the Canopy. Physiologia Plantarum, 138, 289-300.
http://dx.doi.org/10.1111/j.1399-3054.2009.01333.x - 14. Matsuda, R., Ohashi-Kaneko, K., Fujiwara, K. and Kurata, K. (2007) Analysis of the Relationship between Blue-Light Photon Flux Density and the Photosynthetic Properties of Spinach (Spinacia oleracea L.) Leaves with Regard to the Acclimation of Photosynthesis to Growth Irradiance. Soil Science and Plant Nutrition, 53, 459-465.
http://dx.doi.org/10.1111/j.1747-0765.2007.00150.x - 15. Hoenecke, M.E., Bula, R.J. and Tibbitts, T.W. (1992) Importance of “Blue” Photon Levels for Lettuce Seedlings Grown under Red-Light-Emitting Diodes. HortScience, 27, 427-430.
- 16. Douglas, J.S. (1989) Advanced Guide to Hydroponics. Pelham Books/Stephen Greene Press, London.
- 17. He, J., Tan, B.H.G. and Qin, L. (2011) Source-to-Sink Relationship between Green Leaves and Green Pseudobulbs of C3 Orchid in Regulation of Photosynthesis. Photosynthetica, 49, 209-218.
http://dx.doi.org/10.1007/s11099-011-0023-1 - 18. Schreiber, U., Gademann, R., Ralph, P.J. and Larkum, A.W.D. (1997) Assessment of Photosynthetic Performance of Prochloron in Lissoclinum patella in Hospite by Chlorophyll Fluorescence Measurements. Plant and Cell Physiology, 38, 945-951.
http://dx.doi.org/10.1093/oxfordjournals.pcp.a029256 - 19. Rascher, U., Liebig, M. and Lüttge, U. (2000) Evaluation of Instant Light-Response Curves of Chlorophyll Fluorescence Parameters Obtained with a Portable Chlorophyll Fluorometer on Site in the Field. Plant, Cell & Environment, 23, 1397-1405.
http://dx.doi.org/10.1046/j.1365-3040.2000.00650.x - 20. Goins, C.D., Yorio, N.C., Sanwo-Lewandowski, M.M. and Brown, C.S. (1998) Life Cycle Experiments with Arabidopsis under Red Light-Emitting Diodes (LEDs). Life Support and Biosphere Science, 5, 143-149.
- 21. Schuerger, A.C., Brown, C.S. and Stryjewski, E.C. (1997) Anatomical Features of Pepper Plants (Capsicum annuum L.) Grown under Red Light-Emitting Diodes Supplemented with Blue or Far-Red Light. Annals of Botany, 79, 273-282.
http://dx.doi.org/10.1006/anbo.1996.0341 - 22. Gautier, H., Varlet-Grancher, C. and Baudry, N. (1997) Effects of Blue Light on the Vertical Colonization of Space by White Clover and Their Consequences for Dry Matter Distribution. Annals of Botany, 80, 665-671.
http://dx.doi.org/10.1006/anbo.1997.0504 - 23. Saebo, A., Krekling, T. and Appelgren, M. (1995) Light Quality Affects Photosynthesis and Leaf Anatomy of Birch Plantlets in Vitro. Plant Cell, Tissue and Organ Culture, 41, 177-185.
http://dx.doi.org/10.1007/BF00051588 - 24. McCree, K.J. (1972) Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agricultural Meteorology, 9, 191-216.
http://dx.doi.org/10.1016/0002-1571(71)90022-7 - 25. Goto, E. (2003) Effects of Light Quality on Growth of Crop Plants under Artificial Lighting. Environment Control in Biology, 41, 121-132.
http://dx.doi.org/10.2525/ecb1963.41.121 - 26. Furuyama, S., Ishigami, Y., Hikosaka, S. and Goto, E. (2014) Effects of Blue/Red Ratio and Light Intensity on Photomorphogenesis and Photosynthesis of Red Leaf Lettuce. Acta Horticulturae, 1037, 317-322.
- 27. Li, Q. and Kubota, C. (2009) Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environmental and Experimental Botany, 67, 59-64.
http://dx.doi.org/10.1016/j.envexpbot.2009.06.011 - 28. Ohashi-Kaneko, K., Takase, M., Kon, N., Fujiwara, K. and Kurata, K. (2007) Effect of Light Quality on Growth and Vegetable Quality in Leaf Lettuce, Spinach and Komatsuna. Environment Control in Biology, 45, 189-198.
http://dx.doi.org/10.2525/ecb.45.189 - 29. Folta, F.M., Koss, L.L., McMorrow, R., Kim, H.-H., Kenitz, J.D., Wheeler, R. and Sager, J.C. (2005) Design and Fabrication of Adjustable Red-Green-Blue LED Light Arrays for Plant Research. BMC Plant Biology, 5, 17.
http://dx.doi.org/10.1186/1471-2229-5-17 - 30. Terashima, I., Fujita, T., Inoue, T., Chow, W.S. and Oguchi, R. (2009) Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White: Revisiting the Enigmatic Question of Why Leaves Are Green. Plant and Cell Physiology, 50, 684-697.
http://dx.doi.org/10.1093/pcp/pcp034 - 31. Farquhar, G.D. and Sharkey, T.D. (1982) Stomatal Conductance and Photosynthesis. Annual Review of Plant Physiology, 33, 317-345.
http://dx.doi.org/10.1146/annurev.pp.33.060182.001533 - 32. Parkhurst, D.F. (1994) Diffusion of CO2 and Other Gases inside Leaves. New Phytologists, 126, 449-479.
http://dx.doi.org/10.1111/j.1469-8137.1994.tb04244.x - 33. He, J. and Lee, S.K. (2001) Relationship among Photosynthesis, Ribulose-1,5-Bisphosphate Carboxylase (Rubisco) and Water Relations of Subtropical Vegetable Chinese Broccoli Grown in the Tropics by Manipulation of Root-Zone Temperature. Environmental and Experimental Botany, 46, 119-128.
http://dx.doi.org/10.1016/S0098-8472(01)00089-2 - 34. Sharkey, T.D. and Raschke, K. (1981) Effect of Light Quality on Stomatal Opening in Leaves of Xanthium strumarium L. Plant Physiology, 68, 1170-1174.
http://dx.doi.org/10.1104/pp.68.5.1170 - 35. Savvides, A., Fanourakis, D. and van Ieperen, W. (2012) Co-Ordination of Hydraulic and Stomatal Conductances across Light Qualities in Cucumber Leaves. Journal of Experimental Botany, 63, 1135-1143.
http://dx.doi.org/10.1093/jxb/err348 - 36. He, J., Lee, S.K. and Dodd, I.C. (2001) Limitations to Photosynthesis of Lettuce Grown under Tropical Conditions: Alleviation by Root-Zone Cooling. Journal of Experimental Botany, 52, 1323-1330.
http://dx.doi.org/10.1093/jexbot/52.359.1323 - 37. He, J. and Lee, S.K. (2004) Photosynthetic Utilization of Radiant Energy by Temperate Lettuce Grown under Natural Tropical Condition with Manipulation of Root-Zone Temperature. Photosynthetica, 42, 457-463.
http://dx.doi.org/10.1023/B:PHOT.0000046166.29815.94 - 38. Krause, G.H. and Weis, E. (1991) Chlorophyll Fluorescence and Photosynthesis: The Basics. Annual Review Plant Physiology and Plant Molecular Biology, 42, 313-349.
http://dx.doi.org/10.1146/annurev.pp.42.060191.001525 - 39. Schreiber, U. and Klughammer, C. (2013) Wavelength-Dependent photodamage to Chlorella Investigated with a New Type of Multi-Color PAM Chlorophyll Fluorometer. Photosynthesis Research, 114, 165-177.
http://dx.doi.org/10.1007/s11120-013-9801-x - 40. Hemming, S. (2011) Use of Natural and Artificial Light in Horticulture—Interaction of Plant and Technology. Acta Horticulturae, 907, 25-36.
NOTES

*Corresponding author.








