Journal of Materials Science and Chemical Engineering
Vol.1 No.6(2013), Article ID:39822,4 pages DOI:10.4236/msce.2013.16007
Fabrication by Fine Particles and Evaluation of WO3 Photo Semiconductor Electrode
Department of Mechanical Systems Engineering, Faculty of Engineering, Aich University of Technology, Gamagori, Japan
Email: ohtake@aut.ac.jp
Copyright © 2013 Toshihito Ohtake. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 15, 2013; revised October 15, 2013; accepted October 22, 2013
Keywords: Semiconductor; Electrode; WO3; Photocatalysis; Photoelectrochemistry; Water Splitting
ABSTRACT
Application of semiconductor particles has been noticed to solve energy problems as photocatalysis for O2 evolution in water splitting etc. We are trying fabrication of semiconductor electrode by n-WO3 particle toward O2 evolution in water splitting. The electrode obtained high photooxidation properties of water as preventing effective recombination between electrons and holes by utilizing fine semiconductor particles. Particularly, application of suspension prepared by ball milling was able to obtain fine n-WO3 thin film and the remarked semiconductor properties.
1. Introduction
We have noticed that water splitting by the use of a semiconductor electrode or photocatalysis is utilized for alternative energy resources by solar energy. At present, the efficiency is very low in order to absorb only ultraviolet ray for a titanium oxide or other metal oxide etc. as the semiconductors. Therefore, development of photo semiconductor materials is required to absorb broad visible light region toward high efficiency [1-8].
The evaluation has performed photoelectrochemically toward utilization of solar energy [9-14], and we have attempted fabrication a complex semiconductor electrode consisting of n-Si and n-WO3. The n-Si/WO3 semiconductor electrode is expected to absorb long wavelength at n-Si and short wavelength at WO3 in solar lights for water splitting efficiency, which is called for Z-scheme as known to two steps electron transport chain of photosynthesis. At first, we attempted fabrication of highly active n-WO3 electrode on FTO substrate in this study, and the n-WO3 properties was evaluated photoelectrochemically.
2. Methodology
2.1. Photoelectrochemical Measurements
The n-WO3 thin film semiconductor electrode was fabricated by a doctor blade method, and evaluated photoelectrochemically by forming the thin film on FTO substrate. Current-voltage (j-V) measurements of the WO3 electrode was performed by illuminating it with 100 mW/cm2, A.M. 1.5 Xe lamp with imposing anodic bias at 50 mV/s, which was constructed with potentiostat, Ag/ AgCl reference electrode and Pt counter electrode in 0.1 mol/l Na2SO4 electrolyte as shown in Figure 1. Action spectra were measured by 300 W Xe lamp filtered out UV ray (UV-33) through a monochromator.
2.2. Fabrication of WO3/FTO Electrode
WO3 suspension was prepared with the WO3 powder (Wako Pure Chemical Industries, Ltd.) at ca. 200 nm particle size, which was obtained by mixing 1 g WO3 + 0.1 g HNO3 (60%) + 0.2 g H2O and a small amount of surfactant (Triton-X 100, MP Biomedicals, Inc.). The WO3 thin film was formed by applicating the suspension with doctor blade method on FTO substrate, and was calcinated at 450˚C for 30 min in air. Furthermore finer WO3 powder (Aldrich) was also utilized as ca. 30 - 50 nm particle size.
2.3. Fine WO3 Powder Preparation by Ball Milling
A ball milling was applied to prepare a finer WO3 powder with ZrO2 balls at 3 mm diameter. The finer WO3 suspension was produced by mixing 6 g WO3
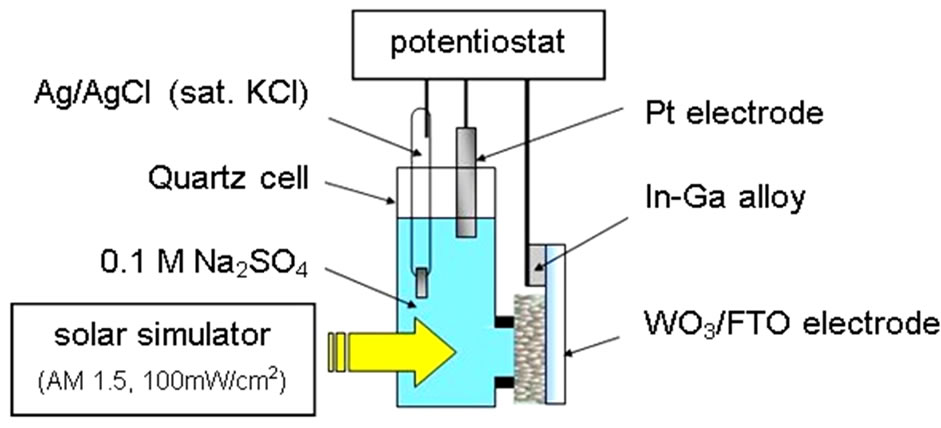
Figure 1. Photoelectrochemical evalutation system to measure n-WO3 semiconductor electrode on the FTO substrate.
powder (Aldrich) + 14 ml H2O + 70 g ZrO2 balls in 30 ml bottle at 24 h in the ball milling. The WO3/FTO electrode was fabricated by applicating the suspension on the FTO substrate at 450˚C for 30 min sintering in air similarly.
3. Results and Discussion
3.1. Photoelectrochemical Properties
The j-V curves were shown about WO3/FTO electrode under dark, illumination and illumination in adding methanol as a reducing agent in Figure 2, which obtained anodic photocurrent, and indicated water photooxidation. The photocurrent in the presence of methanol was about twice and means giving knowledge as following sentences. Photooxidation of methanol was known to two processes for electron transfer at direction and secondary. In the case of the direct process, methanol adsorbed on the WO3 surface reacts with hole [15,16].
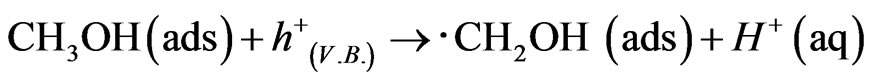
The hole makes photooxidaton of methanol proceed preferentially in comparison to that of water. Hence recombination between electrons and holes is prevented efficiently, and the generation of ∙CH2OH radical caused injection of electrons to conduction band in WO3 to indicate enough negative redox potential E0(∙CH2OH/CH2OH) = −0.97 V in following equation, and double photocurrent is observed.
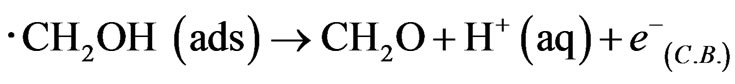
The electron transfer corresponds with the j-V curves in Figure 2. In addition, the secondary electron transfer by electrolyte diffused into WO3 thin film etc. except the photocurrent would not occur not to decrease the photocurrent remarkably in the absence of methanol [15,16]. Consequently, the fabricated WO3/FTO electrode would show good semiconductor properties for water photooxidation.
Furthermore, WO3/FTO electrode based on a finer WO3 powder at 30 - 50 nm diameter (Aldrich) was evaluated as shown in Figure 3. The photocurrent increased

Figure 2. Current-voltage curves about WO3/FTO electrode under dark (black line), illumination (red line) and the illumination in adding a small amount of methanol (dashed blue line) in 0.1 mol/l Na2SO4.
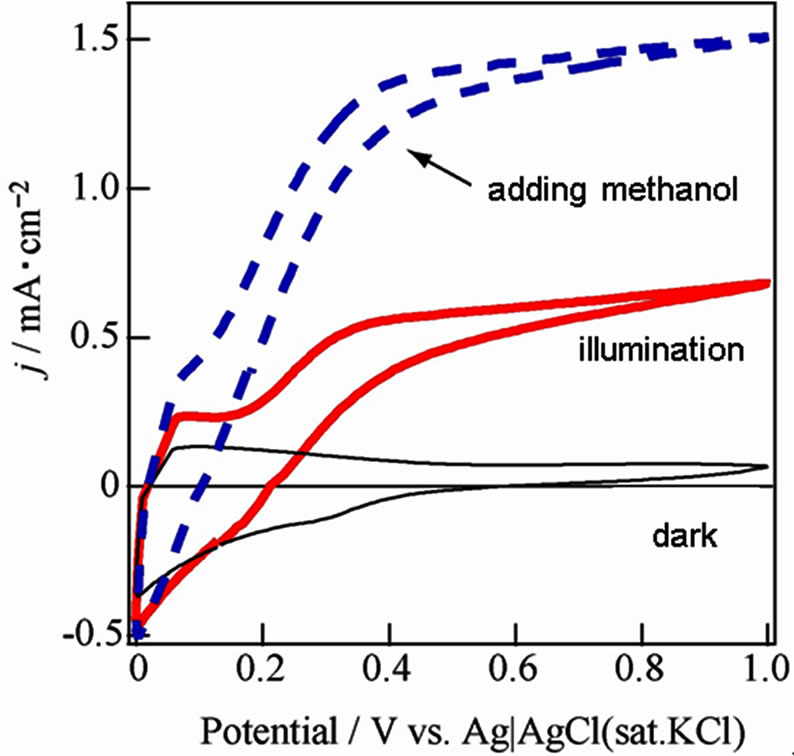
Figure 3. Current-voltage curves about WO3/FTO electrode based on fine WO3 powder under dark (black line), illumination (red line) and the illumination in adding a small amount of methanol (dashed blue line) in 0.1 mol/l Na2SO4.
as compared with that of the WO3/FTO electrode based on the previous WO3 powder, which would suggest that carrier transfer was faster to be deposited with high density and homogeneous. Hence, the finer powder will be required to obtain the excellent semiconductor properties.
3.2. Application of Ball Milling
We attempted fabrication of excellent WO3/FTO electrode by a ball milling to prepare most homogeneous WO3 suspension from the WO3 powder at 30 - 50 nm diameter (Aldrich) without the surfactant. The suspendsion was markedly homogeneous without separation or precipitation. The j-V curves of the WO3/FTO electrode indicated increase of photocurrent in the presence of methanol particularly in Figure 4. The results will suggest that the fabrication method by utilizing the ball milling process of WO3 powder is very effective for the excellent semiconductor properties. Absorbance of the WO3 thin film indicated a large absorption at short wavelength within ca. 440 nm as shown in Figure 5, and corresponded to band gap at 2.8 eV. Moreover, an action spectrum in Figure 6 also accorded with the absorption
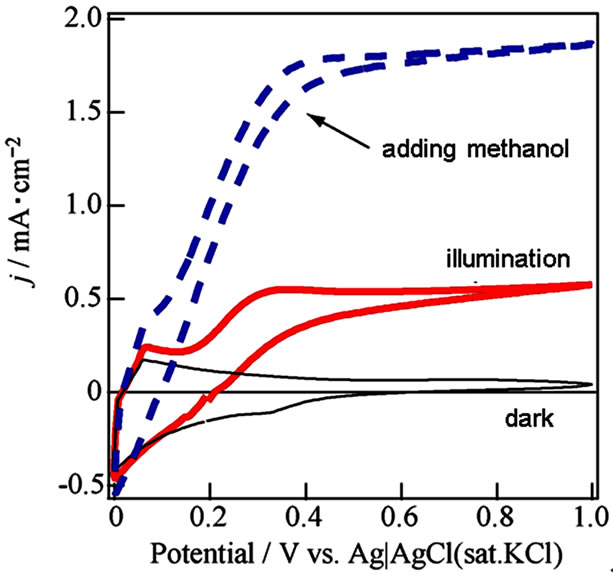
Figure 4. Current-voltage curves about WO3/FTO electrode based on WO3 suspension prepared by ball milling under dark (black line), illumination (red line) and the illuminetion in adding a small amount of methanol (dashed blue line) in 0.1 mol/l Na2SO4.

Figure 5. Absorption spectrum of WO3 thin film fabricated by utilizing ball milling on Kubelka-Munk analysis.

Figure 6. Action spectrum of WO3/FTO electrode fabriccated by utilizing ball milling in 0.1 mol/l Na2SO4.
spectrum, and the results would indicate that the ball milling method was one of valid fabrication methods to prepare the thin film for ideal photo semiconductor properties.
4. Conclusion
Application of the fine WO3 particle improved photoelectrochemical properties, which was more effective by ball milling process for WO3/FTO electrode. These results suggested that the recombination between electrons and holes was prevented and anodic current was improved in the presence of methanol, and would expect for water photo splitting.
REFERENCES
- R. Nakamura and Y. Nakato, “Primary Intermediates of Oxygen Photoevolution Reaction on TiO2 (Rutile) Particles, Revealed by in Situ FTIR Absorption and Photoluminescence Measurements,” Journal of the American Chemical Society, Vol. 126, No. 4, 2004, pp. 1290-1298. http://dx.doi.org/10.1021/ja0388764
- R. Nakamura, T. Tanaka and Y. Nakato, “Mechanism for Visible Light Responses in Anodic Photocurrents at N-Doped TiO2 Film Electrodes,” The Journal of Physical Chemistry B, Vol. 108, No. 30, 2004, pp. 10617-10620. http://dx.doi.org/10.1021/jp048112q
- A. Fujishima and K. Honda, “Electrochemical Photolysis of Water at a Semiconductor Electrode,” Nature, Vol. 238, No. 5358, 1972, pp. 37-38. http://dx.doi.org/10.1038/238037a0
- H. Kato, K. Asakura and A. Kudo, “Highly Efficient Water Splitting into H2 and O2 over Lanthanum-Doped NaTaO3 Photocatalysts with High Crystallinity and Surface Nanostructure,” Journal of the American Chemical Society, Vol. 125, No. 10, 2003, pp. 3082-3089. http://dx.doi.org/10.1021/ja027751g
- G. Hitoki, T. Tanaka, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, “An Oxynitride, TaON, as an Efficient Water Oxidation Photocatalyst under Visible Light Irradiation (λ ≤ 500 nm),” Chemical Communications, Vol. 16, 2002, pp. 1698-1699. http://dx.doi.org/10.1039/b202393h
- M. Hara, G. Hitoki, T. Tanaka, J. N. Kondo, H. Kobayashi and K. Domen, “TaON and Ta3N5 as New Visible Light Driven Photocatalysts,” Catalysis Today, Vol. 78, No. 1-4, 2003, pp. 555-560. http://dx.doi.org/10.1016/S0920-5861(02)00354-1
- W.-J. Chun, A. Ishikawa, H. Fujisawa, T. Tanaka, J. N. Kondo, M. Hara, M. Kawai, Y. Matsumoto and K. Domen, “Conduction and Valence Band Positions of Ta2O5, TaON, and Ta3N5 by UPS and Electrochemical Methods,” The Journal of Physical Chemistry B, Vol. 107, No. 8, 2003, pp. 1798-1803. http://dx.doi.org/10.1021/jp027593f
- A. Ishikawa, T. Takata, J. N. Kondo, M. Hara and K. Domen, “Electrochemical Behavior of Thin Ta3N5 Semiconductor Film,” The Journal of Physical Chemistry B, Vol. 108, No. 30, 2004, pp. 11049-11053. http://dx.doi.org/10.1021/jp048802u
- M. Tripathia, R. Upadhyay and A. Pandey, “Semiconductor Photo-Electrochemical Solar Cells Based on Admixing of Nano-Materials for Renewable Energy,” International Journal of Ambient Energy, Vol. 33, No. 4, 2012, pp. 171-176. http://dx.doi.org/10.1080/01430750.2012.686196
- N. R. de Tacconi, C. R. Chenthamarakshan, G. Yogeeswaran, A. Watcharenwong, R. S. de Zoysa, N. A. Basit and K. Rajeshwar, “Nanoporous TiO2 and WO3 Films by Anodization of Titanium and Tungsten Substrates: Influence of Process Variables on Morphology and Photoelectrochemical Response,” The Journal of Physical Chemistry B, Vol. 110, No. 50, 2006, pp. 25347-25355. http://dx.doi.org/10.1021/jp064527v
- K.-S. Ahn, S.-H. Lee, A. C. Dillon, C. E. Tracy and R. Pitts, “The Effect of Thermal Annealing on Photoelectrochemical Responses of WO3 Thin Films,” Journal of Applied Physics, Vol. 101, No. 9, 2007, pp. 093524-1- 093524-4. http://dx.doi.org/10.1063/1.2729472
- S. Higashimoto, N. Kitahata, K. Mori and M. Azuma, “Photo-Electrochemical Properties of Amorphous WO3 Supported on TiO2 Hybrid Catalysts,” Catalysis Letters, Vol. 101, No. 1-2, 2005, pp. 49-51. http://dx.doi.org/10.1007/s10562-004-3748-7
- A. Tacca, L. Meda, G. Marra, A. Savoini, S. Caramori, V. Cristino, C. A. Bignozzi, V. G. Pedro, P. P. Boix, S. Gimenez and J. Bisquert, “Photoanodes Based on Nanostructured WO3 for Water Splitting,” ChemPhysChem, Vol. 13, No. 12, 2012, pp. 3025-3034. http://dx.doi.org/10.1002/cphc.201200069
- Q. Wang, Z. Wen, Y. Jeong, J. Choi, K. Lee and J. Li, “Li-Driven Electrochemical Properties of WO3 Nanorods,” Nanotechnology, Vol. 17, No. 13, 2006, pp. 3116- 3120. http://dx.doi.org/10.1088/0957-4484/17/13/006
- C. Santato, M. Ulmann and J. Augustynski, “Photoelectrochemical Properties of Nanostructured Tungsten Trioxide Films,” The Journal of Physical Chemistry B, Vol. 105, No. 5, 2001, pp. 936-940. http://dx.doi.org/10.1021/jp002232q
- R. Nakamura, T. Tanaka and Y. Nakato, “Oxygen Photoevolution on a Tantalum Oxynitride Photocatalyst under Visible-Light Irradiation: How Does Water Photooxidation Proceed on a Metal-Oxynitride Surface?” The Journal of Physical Chemistry B, Vol. 109, No. 18, 2005, pp. 8920-8927. http://dx.doi.org/10.1021/jp0501289

