American Journal of Plant Sciences
Vol.5 No.15(2014), Article
ID:47587,10
pages
DOI:10.4236/ajps.2014.515238
Germination of Encholirium spectabile Mart. ex Schult. & Schult. f. Seeds in Response to Temperature and Water Stress
Marlene Feliciano Figueiredo1, Francisco Carlos Barboza Nogueira2*, Charles Lobo Pinheiro2, Selma Freire de Brito2, Sebastião Medeiros Filho2
1University of Vale do Acaraú—UVA, Campus Betânia, City of Sobral, Brazil
2Seed Analysis Laboratory, Department of Phytotecnic, Federal University of Ceará—UFC, Campus do Pici, City of Fortaleza, Brazil
Email: *fcbarbozanogueira@hotmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 10 May 2014; revised 9 June 2014; accepted 26 June 2014
ABSTRACT
A study was conducted to examine the effects of different temperatures and water stress on Encholirium spectabile seeds. We evaluated the germination percentage, speed and time of germination of small (<2.97 mm), medium (2.97 ≤ M ≤ 4.01 mm) and large seeds (>4.01 mm), at temperatures of 15˚C, 20˚C, 25˚C, 30˚C, 35˚C, 40˚C and 45˚C and a photoperiod of 12 hours of light. Seeds were subjected to osmotic potentials of 0, −0.2, −0.4, −0.6, −0.8, −1.0, −1.2 and −1.4 MPa, induced by solutions of polyethylene glycol. The results indicated an excellent germination of medium and large seeds at the temperatures of 20˚C, 25˚C, 30˚C and 35˚C. The temperature of 35˚C provided the best mean germination time of large seeds and a higher speed rate of medium and large seeds. E. spectabile seeds germinate in a wide range of water deficit of −0.2 to −1.2 MPa. Germination reduced at concentrations of −1.0 and −1.2 MPa. No germination occurred at −1.4 MPa. Larger seeds showed the higher germination potential than medium and small seeds at the temperatures of 25˚C, 30˚C and 35˚C and in the range of water deficit of −0.2 to −1.2 Mpa.
Keywords:Seeds Size, Native Species, Osmotic Potential, Polyethylene Glycol, Semiarid Region

1. Introduction
An important feature in plants life history is the capacity of seed germination in semiarid environments, with marked climatic seasonality. In these environments, the plant adopts germination strategies that maximize the chances that are favorable for germination and the development of its seedlings [1] . Nevertheless, germination is a high risk event and it tends to be unpredictable in space and time [2] . Consequently, the way in which a plant species respond to changes in temperature and moisture will increase the likelihood that germination occurs at a time when such conditions are favorable for the establishment and development of its seedlings.
Plants of arid and semiarid regions developed different strategies for survival and reproduction for each of the different stages of their life cycles [3] [4] . In these types of environments, although the seeds are able to resist and persist under extreme conditions of temperature and water stress [4] , moisture scarcity is a major cause of mortality that occurs in seedlings [5] . For this reason, germination occurs during the rainy season short period [6] , and the success of the germination process depends, to a great extend, on water availability in the soil and also on temperature, oxygen, light [7] and on seeds size [8] [9] .
Extreme temperatures and water deficit are the major environmental factors that limit seeds germination and seedling development in regions with marked climatic seasonality [10] . As these restrictions are alleviated by decreasing the temperature and increasing the availability of water, seed responds to these stimuli within the limits set by their genotype [11] . Under this limitation, the water potential expressed by the seed is characteristic of each species and when its value is greater than the substrate water potential, the seed is unable to germinate [7] . Therefore, we should not expect that there will necessarily be a linear relationship between germination, moisture and temperature [12] . Sometimes, seed germination capacity depends on its endogenous limits and on microhabitats local conditions of temperature and moisture for which it has been dispersed [13] [14] . The lack of soil moisture directly affects germination [15] , but when the temperature is closer to the ideal, it causes the water potential to become less limiting for germination [14] .
In certain regions of the northeastern Brazilian semiarid area, bromeliad Encholirium spectabile Mart. ex Schult. & Schult. f. grows fully exposed to the sun. Generally, this bromeliad occurs in inselbergs microhabitats where vegetation presents like rarefied mosaics [16] . The inselbergs are isolated monolithic formations or groups of granitic rocks or gneisses on which a peculiar type of vegetation lives [17] . These rocky “islands” have their own microclimate features and they have a floristic composition which is different from the one in the surrounding areas, whether in wet or semi-arid regions [18] [19] . Besides the lack of water on the substrate of these rocks the air temperature and insolation generally reach average values much higher than those found in their surroundings [17] .
In these inselbergs, E. spectabile is restricted to areas where the substrate on the rocks is poorly developed [17] and, possibly, its seeds take advantage of the rare moments of moisture occurring in these environments in order to germinate. We know that E. spectabile is subjected to unpredictable droughts in these habitats, and in addition, its seeds production events are asynchronous [16] . Therefore, we hypothesized that its seeds germinate in a wide range of water deficit. In this condition, at least some seeds are able to germinate, even in restricted water availability.
On the other hand, in semiarid regions, besides affecting the early seedling development drought can also reduce and delay the germination [20] . In addition, in the short growing season in which temperatures are milder, early or late rains may occur. So, we raise a second hypothesis that larger seeds of E. spectabile germinate in a wide temperature range creating a greater opportunity to generate seedlings with roots that are able to fix them in an undeveloped substrate on the rocks. If this hypothesis is plausible, the prediction is that larger seeds of this bromeliad are able to present a high percentage of germination under different temperatures than medium and smaller seeds.
For this study, we formulated the following research questions: 1) How the seeds of E. spectabile, of unequal size, respond to different and constant temperatures? 2) At what temperature its seeds have the highest percentage and speed of germination and the best mean germination time? 3) What is the range of water stress (generated by PEG 6000) that can be tolerated by their seeds?
2. Material and Methods
2.1. Seeds Collection and Storage Place
Ripe fruits of E. spectabile were collected in August 2012, from three individuals on rocky outcrops located in the municipality of Graça, the northwest region of Ceará. This region lies between the geographical coordinates 4˚02'46''S and 40˚45'10''W, with humid tropical climate and hot humid tropical climate and altitude of 174.8 meters. The average annual temperature ranges from 26˚C to 28˚C and rainfall is concentrated between the months of January to May, with an annual average of 1507.2 mm [21] .
Exsiccates were prepared with whole plants of E. spectabile and deposited in the Herbarium Francisco José de Abreu Matos at University Vale do Acaraú, Ceará, under the number 15,828. After collecting the fruits, the seeds were manually removed and placed in plastic containers. These containers were taken to the Seed Analysis Laboratory, of the Department of Plant Science, at Federal University of Ceará—UFC, where they were stored in a freezer at an average temperature of 8˚C. Then, two tests were assembled and performed in the period from September 2012 to May 2013.
2.2. Focal Species
E. spectabile, Bromeliaceae, C4 plant, locally known as macambira-de-flecha, is restricted to rocky outcrops in certain regions of the northeastern Brazilian semiarid area [16] and to inselbergs of the Atlantic Forest forming mats firmly anchored to the rock by thick roots [17] . It is distributed, in aggregate, on inselbergs and between the clefts of the rocks producing rosettes with leaves up to 60 cm high [16] . Its seeds are of varying sizes, no dormancy, and its germination is epigeal cryptocotylar. Its economic potential is due to the use of its stems and flowers for making handmade decorative objects. In pharmacology, the ethanol extract of E. spectabile has protective activity of the gastric mucosa [22] . E. spectabile stands out regarding the ecological aspect of harboring nests of Xylocopa abbreviata bees on the stems of its inflorescences. The continuous and asynchronous flowering in the population of E. spectabile allows stems to exist in different development stages within the same aggregate; this fact assures the nest supply throughout the year [16] .
2.3. Biometrics and Water Content of the Seeds
The initial characterization was performed by determining the water content, using a hothouse at 105˚C ± 3˚C for 24 hours, according to the method described in the Rules for Seeds Analysis [23] . For biometric determinations, after mixing and homogenization, 100 seeds chosen at random were used for individual measurement with a digital pachymeter. The length was measured from the base to the apex and width was measured at the midline. Data were subjected to descriptive statistical analysis, in which, with the help of Excel application, we calculated arithmetic mean, standard deviation, standard error and coefficient of variation. Three samples of 100 seeds were separated by three size classes: large (seeds with length > 4.01 mm), medium (seed with length ≤ 4.01 mm and ≥2.97 mm) and small (seeds with length < 2.97 mm) and they were again subjected to descriptive statistical analysis.
2.4. Germination under Different Temperature Conditions
In this trial, seeds of three size classes were exposed to constant temperatures of 15˚C, 20˚C, 25˚C, 30˚C, 35˚C, 40˚C and 45˚C, with a variation of ±1˚C and photoperiod of 12 hours of light. For each treatment, four replicates of 50 seeds in a completely randomized design were used. Seeds were distributed over two germitest-type sheets of filter paper and arranged in a Petri dish with a diameter of 9.50 cm. These Petri dishes were germinated in BOD (Biochemical Oxygen Demand) germination chambers. The substrate was moistened with distilled water in the ratio of two and a half times the weight of the paper, and remoistened when necessary. The number of germinated seeds was evaluated every 24 hours. The seeds were considered germinated when they developed the primary root with at least 1 mm long. At the end of the experiment, which lasted fifteen days, germination percentage (%G), germination speed index (GSI) and mean germination time (MGT) were determined according to the following formulas:
% (G) = N/A*100, where N = number of germinated seeds and A = total number of seeds put to germinate;
GSI = G1/N1 + G2/N2 + ...+ Gn/Nn, where:
G1, G2, ... Gn = number of germinated seeds in each day and N1, N2, ... Nn = number of days elapsed since the day of sowing.
MGT = (∑Gi*Ti)/∑G, with the results expressed in days, where:
Gi = number of germinated seeds within a given time interval Ti;
G = number of germinated seeds;
Ti = days of germination.
2.5. Germination under a Water Potential Gradient
For this study, we used seeds of large size class (>4.01 mm) which showed that, in the first trial, a higher percentage of germination at the best temperature (35˚C) and in which the germination process occurred in less time. We used different concentrations of polyethylene glycol (PEG 6000) to evaluate the effect of water stress on seed germination and seedling early development [24] : 0 (control) −0.2 (using 137,003 g/L of distilled water), −0.4 (199,038 g/L of distilled water), −0.6 (246,699 g/L of distilled water), −0.8 (286,899 g/L of distilled water), −1.0 (322,327 g/L of distilled water), −1.2 (354,361 g/L distilled water) and −1.4 (383,823 g/L of distilled water) MPa. PEG 6000 is the most appropriate method to quantify the water available to the seed, as an inert colloidal solution, non-penetrating in the seed and with effects similar to the properties of soil particles matrices [4] . Analyses were carried out in a completely randomized design with four replications of 50 seeds per treatment. The seeds were placed in Petri dishes and kept in a germination chamber (BOD). To minimize the variation of water potential, the Petri dishes were wrapped in a plastic film and weighed every two days in order to replace the evaporated water [25] .
To determine the influence of water stress on the seeds in each treatment, we followed the method proposed by Silveira et al. [26] . The non-germinated seeds were transferred to Petri dishes with distilled water and kept in a germination chamber at a temperature of 35˚C and in a photoperiod of 12 hours, for an additional period of fifteen days. At the end of the experiment, the number of germinated seeds was expressed in percentage of germination.
2.6. Statistical Analysis
We used a multivariate analysis of variance (MANOVA) to evaluate the effects of different treatments on seed germination and seedlings of E. spectabile. Data were analyzed with SPSS 20 for Windows statistical package [27] . Averages that showed statistically significant differences were compared using a Bonferroni adjusted alpha level equal to 0.017. To meet the criterion of normality, the data of germination percentage were transformed into arcsine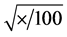 . The treatments with osmotic potential and distilled water (recovery) were submitted to ANOVA (F-test) and treatment means were separated using Fisher’s least significant difference (LSD) tests at the <0.05 level.
. The treatments with osmotic potential and distilled water (recovery) were submitted to ANOVA (F-test) and treatment means were separated using Fisher’s least significant difference (LSD) tests at the <0.05 level.
3. Results
3.1. Influence of Temperature and Seed Weight on Germination
The studied seeds sample of E. spectabile showed difference in size, with the length ranging from 2.22 to 5.27 mm and width ranging from 1.12 to 4.33 mm. Table 1 shows the sample average with its deviations and also the three size classes: small (length: 2.22 to 2.96 mm and width: 1.12 to 2.96 mm), medium (length: 2.97 to 4.01 mm and width: 1.19 to 3.14 mm) and large (length: 4.02 to 5.27 mm, width: 1.98 to 4.33 mm). The weight of thousand seeds was 0.52 grams, with a standard deviation of ±0.007 which allows us to infer that a kilogram of E. spectabile seeds may contain 1,923,076 seeds. The water content of the seeds was 10.62%, 9.70% and 11.25% in the small, medium and large classes, respectively.
A multivariate analysis of variance (MANOVA) indicated a significant effect of temperature (F = 27.843, p < 0.0001; Wilk’s Lambda = 0.010, partial Eta Squared (η2) = 0.681), of the seed size (F = 134.645, p < 0.0001, Wilk’s Lambda = 0.005, partial Eta Squared (η2) = 0.928) and of size × temperature interaction (F = 7.874, p <0.0001; Wilk’s Lambda = 0.029, partial Eta Squared (η2) = 0.587) on the three dependent variables (percentage of germination, germination speed rate and mean germination time).
A dependent variables analysis, by using a Bonferroni adjusted alpha level of 0.017, showed the contribution of temperature, seed size and size × temperature interaction to the three dependent variables (Table 2).
Seeds subjected to treatments with constant the temperatures of 15˚C and 45˚C, with 12 hours of light/12 hours of darkness did not germinate. Therefore, these temperatures were not included for analysis purpose. The five temperatures studied influenced the germination of E. spectabile seeds regarding germination percentage, germination speed rate and mean germination time (Tables 3-5). For seeds of smaller size (<2.97 mm), the five temperatures studied similarly affected the germination percentage. The excellent germination of E. spectabile seeds was achieved at the temperatures of 20˚C, 25˚C and 35˚C, conditions that promoted a high value of germination percentage to seeds of large (length > 4.01 mm) and medium sizes (2.97 ≤ M ≤ 4.01 mm). Despite
Table 1 . Average, standard deviation, coefficient of variation and confidence interval regarding biometric determinations (length and width) and weight in a sample of 100 seeds of Encholirium spectabile and also three samples of 100 seeds of three size classes: small (S < 2.97 mm), medium (2.97 ≤ M ≤ 4.01 mm) and large (L > 4.01 mm).
Table 2. Results of the analysis of variance (ANOVA) by using a Bonferroni adjusted alpha level of 0.017 on germination percentage, germination speed rate and mean germination time of Encholirium spectabile at temperatures of 20˚C, 25˚C, 30˚C, 35˚C and 40˚C, size classes: large (L > 4.01 mm), medium (2.97 ≤ M ≤ 4.01 mm) and small (S < 2.97 mm) and size × temperature interaction.
this result, the temperature of 35˚C was used in conducting the experiment of water stress because it promoted the best mean germination time for medium and large seeds and the largest germination speed rate for medium and large seeds.
Table 3. Untransformed final germination percentage between brackets and transformed germination percentage (mean ± standard deviation) of Encholirium spectabile large (L > 4.01 mm), medium (2.97 ≤ M ≤ 4.01 mm) and small seeds (S < 2.97 mm) at temperatures of 20˚C, 25˚C, 30˚C, 35˚C and 40˚C.
Means followed by the same lowercase letters (lines) and capital letters (columns) are not statistically different from each other, at the Bonferroni adjusted alpha level of 0.017.
Table 4. Germination speed rate (GSR) (means ± standard deviation) of Encholirium spectabile large (L > 4.01 mm), medium (2.97 ≤ M ≤ 4.01 mm) and small seeds (S < 2.97 mm) at the temperatures of 20˚C, 25˚C, 30˚C, 35˚C and 40˚C.
Means followed by the same lowercase letters (lines) and capital letters (columns) are not statistically different from each other, at the Bonferroni adjusted alpha level of 0.017.
Table 5. Mean Germination Time (MGT) (means ± standard deviation) of Encholirium spectabile large (L > 4.01 mm), medium (2.97 ≤ M ≤ 4.01 mm) and small seeds (S < 2.97 mm) at the temperatures of 20˚C, 25˚C, 30˚C, 35˚C and 40˚C.
Means followed by the same lowercase letters (lines) and capital letters (columns) are not statistically different from each other, at the Bonferroni adjusted alpha level of 0.017.
3.2. Influence of Water Stress on Germination
The germination percentage was significantly influenced by different treatments with polyethylene glycol (PEG 6000), (F = 58.03, P < 0.001) and by the recovery experiment (F = 165.70, P < 0.001). Seed germination was not affected by the concentration of PEG 6000 up to −0.8 MPa. There was a reduction in the germination at the concentrations of −1.0 and −1.2 MPa. No seeds germination was observed at −1.4 MPa. After E. spectabile seeds transfer to distilled water, germination percentage (recovery) did not differ from control except for the concentrations from −0.2 to −0.6 MPa (Table 6).
4. Discussion
The heterogeneity observed in the size of E. spectabile seeds was due to the position of the seeds in the fruit. The loculicide, trilocular and polispermic capsule-type fruit presents conical shape with a wide base with sharp narrowing in the upper third, which houses the smallest seeds. This variation in seed size was also recorded for Dyckia goehringii (Bromeliaceae) due to its position in the fruit [28] . The position of the seed in the fruit can influence its physiological quality. A study conducted with species Albizia saman, Cassia fistula, Cassia hybrida and Delonix regia revealed that seeds taken from the final portion of the pod in D. regia and from the middle portion of the fruits of the other three species mentioned, showed seeds with higher vigor and seedling establishment [29] . The E. spectabile seeds of medium and large size showed greater vigor than smaller ones, that is why they can be indicated for the multiplication of the species.
The small, medium and large E. spectabile seeds size classes showed similar water content (10.62%, 9.70% and 11.25%, respectively), within the limits for species with orthodox seeds. This characteristic favors the germination viability of the species, since their seeds are kept in appropriate storage conditions. In general, seeds which have water contents between 10% and 12% assure the maintenance of germination for a period of six to eight months [28] [30] .
The size of seeds in E. spectabile significantly affected its germination. Medium and large seeds showed higher germination compared to small seeds. This superiority is probably due to the fact that they showed larger embryos and reserves enough to promote greater vigor in these size classes [31] .
Although the expected minimum temperature for germination of seeds of tropical species is between 10˚C and 15˚C and the maximum one is around 40˚C [32] , temperatures of 15˚C and 45˚C interfered with the metabolism of E. spectabile seeds preventing its germination. The temperature of 15˚C should be the minimum temperature at which the species triggers the delay of its metabolic rates, while the other extreme (45˚C) denatures its proteins [33] . However, this is not the standard for Bromeliads from other regions. Seeds of Dyckia tuberosa (Vell.) Beer, a terrestrial bromeliad of the Cerrado (savannah) germinated between 15˚C and 40˚C [34] . Seeds of two sandspit bromeliads, Aechmea nudicaulis (L.) and Streptocalyx floribundus (Martius ex Schultes f.) Mez, reached maximum germination temperature between 40˚C and 45˚C, although low germination occurs in this temperature range [35] . Pereira et al. [36] studied the germination behavior of four species of Bromeliaceae, Alcantarea imperialis (Rupestrian field), Pitcairnia flammea (Rupestrian field), Vriesea heterostachys (Atlantic Forest) and Vriesea penduliflora (Atlantic Forest) derived from Ibitipoca State Park, Minas Gerais. The seeds of A. imperialis, V. Heterostachys and V. penduliflora were able to germinate in a wide range of temperatures between 15˚C and 35˚C, except for V. penduliflora which was also able to germinate at 10˚C.
Table 6. Percentage of final germination (mean ± standard deviation) of Encholirium spectabile seeds in different concentrations of PEG 6000 and recovery experiment.
Means followed by the same letters (columns) are not statistically different from each other by Fisher’s LSD (P = 0.05).
Seeds of E. spectabile, unequal in size, showed different responses regarding germination percentage when subjected to the constant temperatures analyzed. The prediction that their larger seeds would show higher germination percentage than medium and small seeds was confirmed at the temperatures of 20˚C, 25˚C and 35˚C. Seeds of Dyckia goehringii Gross & Rauh, a native bromeliad of Cerrado, were evaluated on a sheet of paper similar to E. spectabile at temperatures of 20˚C, 25˚C, 30˚C and 35˚C by [28] . The results obtained by these authors showed that the seeds of D. goehringii do not present an impediment to water absorption which is influenced by its size.
Large and medium seeds expressed higher germination speed rate at 35˚C, indicating that, at this temperature, E. spectabile seeds have greater vigor. According to Duarte et al. [28] D. goehringii of large seeds have higher germination and vigor, generating more vigorous seedlings than small seeds, expressing its best physiological potential at the temperature of 30˚C. On the other hand, the mean germination time did not show a variable indicated to examine the influence of mass in E. spectabile seeds, as there was no significant difference between small, medium and large seeds at the temperatures of 30˚C, 35˚C and 40˚C.
E. spectabile seeds germinate in a wide range of water deficit of −0.2 to −1.2 MPa. It is possible that this is one of its evolutionary strategies to acquire water supplies needed to germinate and to survive in the substrate with water restrictions. In semi-arid regions, where rainfall is irregular and uncertain in time and space, this species should take advantage of the short periods of rain to promote its own seed germination [6] .
Soil moisture or habitat is one of the major abiotic factors that affect seed germination [15] . In the case of E. spectabile seeds, no germination was observed when the osmotic potential reached −1.4 MPa. This must be the osmotic potential characteristic of the species when the seed is unable to germinate [7] .
Although they showed a low germination percentage (36.28% and 30.38%), E. spectabile seeds were able to germinate when exposed to osmotic potentials of −1.0 and −1.2 MPa. This may also be another strategy used by the species to take advantage of low water availability in its habitat. The characteristic of seeds asynchronous dispersion [16] and the ability to germinate in wide range of water deficit can be vital to seedlings of E. spectabile and decisive for the recruitment of individuals in environments with water restrictions [37] .
On the other hand, the results found for the germination of E. spectabile seeds reveal that the reduced germination in osmotic potentials −1.0 and −1.2 MPa can reduce the fitness of their seedlings. Consequently, it can reduce the likelihood of individuals to survive and reach adult stage. These predictions are consistent with other studies on woody species in semi-arid regions. Prosopis caldenia seeds germinate and grow under water stress conditions. However, the availability of water in the soil limits the establishment, survival and growth to adult stage [10] .
5. Conclusion
E. spectabile seeds of unequal sizes respond differently to the influence of temperature on germination and to water stress. Larger seeds have higher germination than medium and small seeds at the temperatures of 20˚C, 25˚C and 35˚C and in the range of water deficit of −0.2 to −1.2 MPa. Positive response of E. spectabile seed germination at different temperatures and wide range of water stress can be one of the strategies used in the establishment and reproduction of the species in the rocky habitat of semiarid region.
References
- Poorter, L., Bongers, F., Sterck, F.J. and Woll, H. (2005) Beyond the Regeneration Phase: Differentiation of Height-Light Trajectories among Tropical Tree Species. Journal of Ecology, 93, 256-267. http://dx.doi.org/10.1111/j.1365-2745.2004.00956.x
- Du, Y. and Huang, Z. (2008) Effects of Seed Mass and Emergence Time on Seedling Performance in Castanopis chinensis. Forest Ecology and Management, 255, 2495-2501. http://dx.doi.org/10.1016/j.foreco.2008.01.013
- Chesson, P., Gebauer, R.L.E., Schwinning, S., Huntly, N., Wiegand, K., Ernest, S.K.M., Sher, A., Novoplansky, A. and Weltzin, J.F. (2004) Resouce Pulses, Species Interactions and Diversity Maintenance in Arid and Semi-Arid Environments. Oecologia, 141, 236-253. http://dx.doi.org/10.1007/s00442-004-1551-1
- Gorai, M., Tlig, T. and Neffati, M. (2009) Influence of Water Stress on Seed Germination Characteristics in Invasive Diplotaxis harra (Forssk.) Boiss (Brassicaceae) in Arid Zone of Tunisia. Journal of Phytology, 1, 249-254. http://journal-phytology.com/index.php/phyto/article/view/731/615
- Moles, A.T. and Westoby, M. (2004) What Do Seedlings Die From, and What Are the Implications for Evolution of Seed Size? Oikos, 106, 193-199. http://dx.doi.org/10.1111/j.0030-1299.2004.13101.x
- Gorai, M. and Neffati, M. (2007) Germination Responses of Reaumuria vermiculata to Salinity and Temperature. Annals of Applied Biology, 151, 53-59. http://dx.doi.org/10.1111/j.1744-7348.2007.00151.x
- Bewley, J.D. and Black, M. (1994) Seeds: Physiology of Development and Germination. Plenum Press, New York. http://dx.doi.org/10.1007/978-1-4899-1002-8
- Leishman, M.R. and Westoby, M. (1994) The Role of Seed Size in Seedling Establishment in Dry Soil Conditions— Experimental Evidence from Semi-Arid Species. Journal of Ecology, 82, 249-258. http://dx.doi.org/10.2307/2261293
- Leishman, M.R., Wright, I.J., Moles, A.T. and Westoby, M. (2000) The Evolutionary Ecology of Seed Size. In: Fenner, M., Ed., Seeds—The Ecology of Regeneration in Plant Communities, Wallingford, UK, 31-58. http://dx.doi.org/10.1079/9780851994321.0031
- Villalobos, A.E. and Peláez, D.V. (2001) Influences of Temperature and Water Stress on Germination and Establishment of Prosopis caldenia Burk. Journal of Arid Environments, 49, 321-328. http://dx.doi.org/10.1006/jare.2000.0782
- Verslues, P.E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J. and Zhu, J.K. (2006) Methods and Concepts in Quantifying Resistance to Drought, Salt and Freezing, Abiotic Stresses That Affect Plant Water Status. The Plant Journal, 45, 523-539. http://dx.doi.org/10.1111/j.1365-313X.2005.02593.x
- Bonvissuto, G.L. and Busso, C.A. (2007) Germination of Grasses and Shrubs under Various Water Stress and Temperature Conditions. Pyton, 76, 119-131. http://www.revistaphyton.fund-romuloraggio.org.ar/vol76/bonvissutoSeedrain.pdf
- Baskin, C.C. and Baskin, J.M. (1988) Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination. Academic Press, New York.
- Qi, M.Q. and Redmann, R.E. (1993) Seed Germination and Seedling Survival of C3 and C4 Grass under Water Stress. Journal of Arid Environments, 24, 277-285. http://dx.doi.org/10.1006/jare.1993.1024
- Pratap, V. and Sharma, Y.K. (2010) Impacto of Osmotic Stress on Seed Germination and Seedling Growth in Black Gram (Phaseolus Mungo). Journal of Environmental Biology, 31, 721-726. http://www.jeb.co.in/journal_issues/201009_sep10/paper_29.pdf
- Ramalho, M., Batista, M.A. and Silva, M. (2004) Xylocopa (Monoxylocopa) abbreviata Hurd & Moure (Hymenoptera: Apidae) e Encholirium spectabile (Bromeliaceae): Uma Associação Estreita no Semi-árido do Brasil Tropical. Neotropical Entomology, 33, 417-425. http://dx.doi.org/10.1590/S1519-566X2004000400004
- Porembski, S. (2007) Tropical Inselbergs: Habitat Types, Adaptive Strategies and Diversity Patterns. Revista Brasileira de Botanica, 30, 579-586. http://dx.doi.org/10.1590/S0100-84042007000400004
- Ibisch, P.L., Rauer, G., Rudolph, D. and Barthlott, W. (1995) Floristic, Biogeographical, and Vegetational Aspects of Pre-Cambrian Rock Outcrops (Inselbergs) in Eastern Bolivia. Flora, 190, 299-314.http://agris.fao.org/agris-search/search/display.do
- Porembski, S., Fischer, E., Biedinger, N. (1997) Vegetation of Inselbergs, Quarzitic Outcrops and Ferricretes in Rwanda and Eastern Zaire (Kivu). Bulletin du Jardin Botanique National de Belgique, 66, 81-99. http://dx.doi.org/10.2307/3668138
- Li, H., Li, X., Zhang, D., Liu, H. and Guan, K. (2013) Effects of Drought Stress on the Seed Germination and Early Seedling Growth of the Endemic Desert Plant Eremosparton songoricum (Fabaceae). EXCLI Journal, 12, 89-101. http://www.excli.de/vol12/Li_Zhang_04022013_proof.pdf
- Ipece. Instituto de Pesquisa e Estratégia Econômica do Ceará (2009) Perfil básico municipal: Graça. Disponível. http://www.ipece.ce.gov.br/
- Carvalho, K.I.M., Bernardes, H.B., Machado, F.D.F., Oliveira, I.S., Oliveira, F.A., Nunes, P.H.M., Lima, J.T., Almeida, J.R.G.S. and Oliveira, R.C.M. (2010) Antiulcer Activity of Ethanolic Extract of Encholirium spectabile Mart. ex Schult & Schult f. (Bromeliaceae) in Rodents. Biological Research, 43, 459-465. http://dx.doi.org/10.4067/S0716-97602010000400011
- Brazil (2009) Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento, Brasília.
- Hardegree, S.P. and Emmerich, W.E. (1994) Seed Germination Response to Polyethylene Glycol Solution Depth. Seed Science and Technology, 22, 1-7. http://naldc.nal.usda.gov/download/6952/PDF
- Ishikawa, S.I. and Kachi, N. (2000) Differential Salt Tolerance of Two Artemisia Species Growing in Contrasting Coastal Habitats. Ecological Research, 15, 241-247. http://dx.doi.org/10.1046/j.1440-1703.2000.00345.x
- Silveira, D.G., Pelacani, C.R., Antunes, C.G.C., Rosa, S.S., Souza, F.V.D. and Santana, J.R.F. (2011) Resposta Germinativa de Sementes de Caroá [Neoglaziovia variegata (Arruda) Mez]. Ciência e Agrotecnologia, 35, 948-955. http://www.scielo.br/pdf/cagro/v35n5/a12v35n5.pdf
- SPSS Inc. (2011) SPSS Version 20 (Computer Program). SPSS Inc., Chicago.
- Duarte, E.F., Carneiro, I.F., Silva, N.F. and Guimarães, N.N.R. (2010) Características Físicas e Germinação de Sementes de Dyckia goehringii Gross & Rauh (Bromeliaceae) sob Diferentes Temperaturas. Pesquisa Agropecuária Tropical, 40, 422-429. http://www.revistas.ufg.br/index.php/pat/article/viewarticle/6037
- Srimathi, P., Swaminathan, C., Sivagnaram, K. and Surendran, C. (1991) Seed Attributes in Relation to Their Position in the Pod and Its Influence on Seedling Establishment of Four Ornamental Tree Species. Journal of Tropical Forest Science, 4, 245-248. http://myais.fsktm.um.edu.my/8869/1/1.pdf
- Marcos Filho, J. (2005) Fisiologia de Sementes de Plantas Cultivadas. Fealq, Piracicaba.
- Easton, L.C. and Kleindorfer, S. (2008) Interaction Effects of Seed Mass and Temperature on Germination in Australian Species of Frankenia (Frankeniaceae). Folia Geobotanica, 43, 383-396. http://dx.doi.org/10.1007/s12224-008-9021-x
- Borghetti, F. (2005) Temperaturas Extremas e a Germinação das Sementes. In: Nogueira, R.J.M.C., Araújo, E.L., Willadino, L.G. and Cavalcante, U.M.T., Eds., Estresses Ambientais: Danos e Benefícios em Plantas, MXM Gráfica e Editora, Recife, 207-218.
- Martins, L.D., Costa, F.P., Lopes, J.C. and Rodrigues, W.N. (2012) Influence of Pre-Germination Treatments and Temperature on the Germination of Crambre Seeds (Drambre abyssinica Hochst). Idesia, 30, 23-28. http://www.scielo.cl/scielo.php?pid=S0718-34292012000300003
- Vieira, D.C.M., Socolowski, F. and Takaki, M. (2007) Germinação de Sementes de Dyckia tuberose (Vell.) Beer (Bromeliaceae) sob Diferentes Temperaturas em Luz e Escuro. Revista Brasileira de Botancia, 30, 183-188. http://dx.doi.org/10.1590/S0100-84042007000200003
- Pinheiro, F. and Borghetti, F. (2003) Light and Temperature Requirements for Germination of Seeds of Aechmea nudicaulis (L.) Griesebach and Streptocalyx floribundus (Martius ex Schultes F.) Mez (Bromeliaceae). Acta Botanica Brasilica, 17, 27-35. http://dx.doi.org/10.1590/S0102-33062003000100003
- Pereira, A.R., Andrade, A.C.S., Pereira, T.S., Forzza, R.C. and Rodrigues, A.S. (2009) Comportamento Germinativo de Espécies Epífitas e Rupícolas de Bromeliaceae do Parque Estadual do Ibitipoca. Revista Brasileira de Botanica, 32, 827-838. http://www.scielo.br/pdf/rbb/v32n4/a20v32n4.pdf
- Evans, C.E. and Etherington, J.R. (1990) The Effect of Water Potential on Seed Germination of Some British Plants. New Phytologist, 115, 539-548. http://dx.doi.org/10.1111/j.1469-8137.1990.tb00482.x
NOTES

*Corresponding author.


