Open Journal of Internal Medicine
Vol.1 No.2(2011), Article ID:7699,4 pages DOI:10.4236/ojim.2011.12009
Prevalence of occult hepatitis C in Egyptian patients with non alcoholic fatty liver disease
![]()
1Departments of Endemic Medicine and Hepatology, Cairo University, Cairo, Egypt;
2Medical Biochemistry, Faculty of Medicine, Cairo University, Cairo, Egypt;
3Pathology, Theodor Bilharz Institute, Cairo, Egypt;
4Viral Hepatitis Research Laboratory, National Hepatology and Tropical Medicine Research Institute, Cairo, Egypt.
Email: *dr_ysaad99@yahoo.com
Received 12 June 2011; revised 15 July 2011; accepted 10 August 2011.
Keywords: Genotype 4; Liver Enzymes; NAFLD; Occult HCV
ABSTRACT
This study aim is to assess the prevalence of occult HCV infection among Egyptian patients with non alcoholic fatty liver disease (NAFLD) with elevated AST and ALT, and to correlate presence of occult HCV with severity of liver disease. Patients and Methods: After informed consent 27 patients with elevateed liver enzymes diagnosed as NAFLD were examined for demographic, clinical, laboratory data and Ultrasonography. Liver biopsy was done and tested for HCV RNA in tissue. Genotyping using RFLP analysis of PCR products in the 5’NCR was done for positive cases. Results: HCV RNA in tissue was positive in 11/27 patients (40.7%); genotype was 4a in all positive cases. AST and ALT values showed significantly lower values in occult HCV than the non HCV NAFLD group. Liver biopsy of studied patients showed no significant difference as regard inflammation and fibrosis according to METAVIR score. Conclusions: Occult HCV is highly prevalent among Egyptian NAFLD patients. It seems to induce a mild liver disease. Patients with elevated ALT and negative HCV RNA in sera might be investigated for tissue HCV RNA. Follow up is recommended for the occult HCV patients to monitor progression to overt disease.
1. INTRODUCTION
In most patients with persistent abnormal liver biochemistry, clinical data and serological tests allow identification of the causative factor or disease entity responsible for liver damage. However, in some subjects with persistent alteration of liver enzymes the cause of the disturbance cannot be established on the basis of these clinical and analytical data. These cases are referred to as cryptogenic chronic liver disease, later on non alcoholic fatty liver disease (NAFLD) and non alcoholic steatohepatitis (NASH) represented part of these cases [1].
Some authors have reported that occult hepatitis B virus could be the cause of a proportion of these cryptogenic chronic hepatitis cases [2,3], but no conclusive results have been yielded.
In January 2004, the role of occult hepatitis C virus (HCV) infection in chronic liver disease was first described by Castillo et al. [4]. Patients with elevated liver function tests and negative HCV antibodies and serum RNA may have detectable HCV RNA in their liver tissue and PBMCs. Occult HCV infection is associated with worse activity and fibrosis scores on liver biopsy [4].
In a country like Egypt, which has the highest prevalence of hepatitis C virus (HCV) in the world, ranging from 6% to 28% with an average of approximately 15% in the general population [5-8], and genotype 4 represents over 90% of Egyptian cases [9], with no data about occult HCV. We therefore investigated the prevalence of occult HCV among patients diagnosed as NAFLD with negative HCV Ab and HCV RNA in serum and its effect on the liver disease severity.
2. PATIENTS AND METHODS
After getting informed written consent from 27 patients referred to the Hepatology Unit in Cairo University with elevated transaminases, bright liver on abdominal ultrasonography and negative HCV Ab, all patients have been subjected to a complete history taking including; drugs, alcohol intake, blood transfusion, tattoos, body piercing, sexual behavior. A complete physical examination was performed on each subject includes general examination, estimation of BMI and stigmata of chronic liver disease.
Venous blood samples were drawn after an overnight 12-hour fasting to determine the levels of serum ALT, AST, albumin, prothrombin time, total lipid profile, fasting blood glucose, ANA, ASMA, AMA, HBsAg, HBcAb total, HCV Ab, HCV RNA by PCR Reverse Transcription and Nested Polymerase Chain Reaction [10].
Ultrasound examination: Using a Toshiba machine with a 3.5 MHZ convex probe, patients were examined after at least 8 hours fasting in the supine, right and left lateral positions. Scanning was done through several longitudinal, oblique and transverse scans. Measurements were taken in quiet respiration.
After checking the coagulation profile of the patients and after an overnight fasting; liver biopsy was taken under sonographic guidance, using Tru-cut needles (Baxter Healthcare) (16G, internal diameter 1.6 mm). It was fixed in 10% formalin and paraffin-embedded, and sectioned by microtome with a thickness of 5 µm for routine histological diagnosis and for in situ hybridization. Stained with hematoxylin-eosin and Mason trichrome and scored by an experienced hepatopathologist according to METAVIR score [11].
RNA Extraction from liver tissue: The Nucleic acid extraction was done using QIAamp viral RNA kit then HCV RNA was tested by RT-PCR using primers from the 5_ noncoding region (5_NCR) of the HCV genome [10].
Reverse Transcription and Polymerase Chain Reaction: The protocol for RT-PCR to detect HCV RNA was performed according to Abdel-Hamid et al., 1997 with modifications to increase the sensitivity of the assay. The RT-PCR was carried in a total volume of 100µl containing 1X Taq buffer with 1.5 mM MgCl2 (Roche Molecular Biochemicals, Mannheium, Germany), 0.2 mM dNTPs (Promega Madison, WI, USA), 20 pmole of each primers P1 (5’ GTGAGGAACTACTGTCTTCACGCAG 3’) and P2 (5’ TGCTCATGGTGCACGGTCTACGAGA 3’), 20 units of Human Placental Ribonuclease Inhibitor (HPRI) (RNasin) (Promega Madison, WI, USA), 10 units of AMV Reverse Transcriptase (RT) (Promega Madison, WI, USA), and 2.5 units Taq DNA polymerase (Roche Molecular Biochemicals, Mannheium, Germany). 45µl of master mix was added to each sample and the mixture was incubated at: 42˚C for 30 min for RT (one cycle), 95˚C for 4 min (one cycle), followed immediately by 35 cycles of the following conditions, 94˚C denaturation for 1 minute, 50˚C annealing for 1 minute, 72˚C extension for 1 minute; A final cycle of 72˚C for 10 minutes, all PCR reactions were carried out on GeneAmp PCR Systems 9700; Applied Biosystems. Nested PCR was performed by the transfer of 10 µl of the first PCR product to 90 µl of the second master mix containing 1X Taq buffer (Roche Diagnostics), 0.2 mM dNTP’s (Promega), 20 pmole of each nested primer P3 (5’ TTCACGGCAGAAAGCAGTCTAG 3’) and P4 (5’ CTATCAGGCAGTACCACAAGG 3’), 2.5 units Taq polymerase (Roche Diagnostics). The samples were incubated for 35 cycles as in first round of PCR but without the initial RT step. PCR products were visualized by electrophoresis on ethidium bromide stained 3% agarose gel in 0.5X TBE buffer (GIBCO-BRL, Life Technologies, Gaithersburg, MD, USA).
HCV genotyping by RFLP analysis: Restriction fragment length polymorphism (RFLP) is a method for identifying the six major genotypes of HCV (1a, 1b, 2a, 2b, 3a, 4, 5, 6) by restriction endonuclease cleavage of sequences. These sequences were amplified by nested RT-PCR from the 5’ non-coding region, 10 µl from the nested PCR products of 237 bp, were digested by restriction endonuclease enzymes for 2 hours at 37˚C by both MvaI/HinfI in buffer H and RsaI/HaeIII in buffer L (Boehringer Manheim, Germany) [12,13] in a total volume of 20 µl. Electrophoresis was done in a 4 % Metaphore agarose gel in 0.5X Tris borate buffer (TBE) buffer to separate different specific sequences (FMC Bio products, USA). Bands corresponding to specific 5’ NCR sequences were visualized under UV light and identified according to specific recognition sequence.
3. STATISTICAL METHODS
Descriptive data is presented for all enrolled patients. Quantitative variables presented by mean and standard deviation (SD). Qualitative variables are presented by number and percent. The statistical analyses were performed by non parametric tests (SPSS program v9.0).
4. RESULTS
Out of 27 patients diagnosed as NAFLD, 11 patients (40.7%) had positive HCV RNA in tissue (occult HCV infection). HCV genotyping showed that HCV in the tested samples belongs to genotype 4a. Both groups were matched in age, sex and BMI with no statistical difference as well as the residence or history of past exposure to schistisomiasis (Table 1).
The only statistical significant biochemical parameter was liver transaminases in which AST & ALT were higher in non HCV NAFLD patients (p-value 0.015 & 0.047) respectively (Table 2). Regarding histopathological features, 10/11 (90.9%) in Occult HCV group had A0/F0 while in NAFLD group 12/16 (75%) had A0/F0 with no significant difference (Table 3).
5. DISCUSSION
Egypt has the highest worldwide prevalence of hepatitis C virus (HCV), with an estimated 8 - 10 million among a population of 75 million having been exposed to the virus and 5 - 7 million active infections [14]. Despite the

Table 1. Demographic and clinical features of occult C and NAFLD patients.
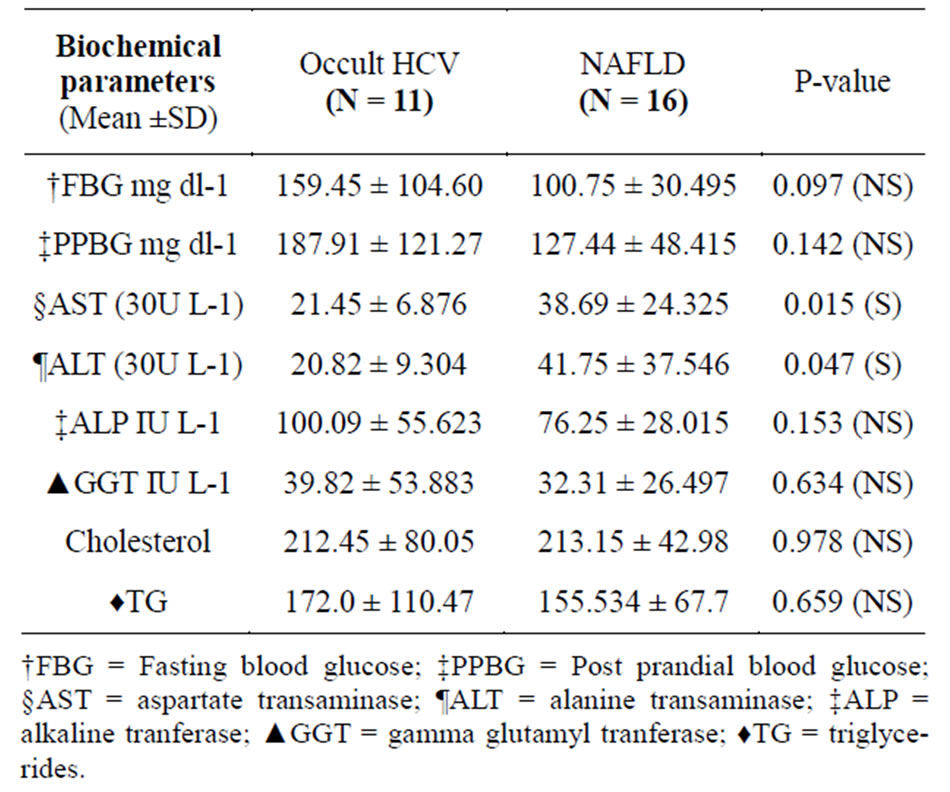
Table 2. Biochemical parameters of occult HCV and NAFLD patients.

Table 3. Histopathological features of occult C and NAFLD patients.
high prevalence of HCV in Egypt, studies have suggested that the epidemic pattern of type 4a infection in Egypt was more recent and quite different from the endemic pattern in sub-Saharan Africa [15,16]. Thus, it seems that the current status of HCV in Egypt is not only a consequence of the mass anti-schistosomal therapy but also due to new infections acquired beyond that era, given that HCV currently represents more than 13% of the annually reported acute hepatitis cases [17].
Abnormal results on liver-function tests have various causes, and the etiology of the liver damage can be established in most cases. However, the etiology of long-standing abnormal results on liver-function tests in some patients is unknown after rigorous exclusion of all known causes of liver diseases until January 2004 when occult hepatitis C virus (HCV) infection was first described [4]. Occult HCV infection is characterized by the presence of HCV-RNA in the liver in the absence of serological markers of infection (anti-HCV and serum HCV-RNA negative) [18,19]. In the current study, 11 patients (40.7%) were found to have occult HCV infection; this incidence comes in context with what have been reported by Castillo in January 2004 who reported 57% incidence of occult HCV infection in chronic liver disease in a study which included 100 patients with persistently long standing abnormal liver function test results. In addition, (84%) of these patients with occult HCV infection also had the antigenomic HCV-RNA strand in the liver tissue, indicating an ongoing viral replication [4]. A similar high rate of Occult HCV infection was also found in an Egyptian study in which14 out of 30 patients with cryptogenic hepatitis (46.6%) had intrahepatic HCV RNA. out of 14 patients with intrahepatic HCV RNA, 11 had identifiable viral RNA in PBMCs (78.5%) [20]. This finding may support hypothesis that testing for HCV RNA in liver specimens is considered the gold standard and because liver biopsy is an invasive procedure, HCV RNA detectability was investigated in PBMCs in some studies.
The most common age of occult HCV infection is from 22 - 66 years. Our results were in agreement with this statement yet come in contrast with male predominance that was reported by previous studies [4,21].
In identifying the risk factors of HCV seropositivity in Egypt, our results are in accordance with previous studies [22], in which multivariate regression was used to estimate independent effects of risk factors on HCV seropositivity. Independent risk factors that were insignificant to anti-HCV positivity in adults included: history of, or active infection with, Schistosoma mansoni [22], also neither history of diabetus nor residence are considered a risk factors of occult HCV.
Occult HCV infection is a mild disease, the current study support this hypothesis by showing no significant association between the detection of occult HCV infection and the main symptoms of the studied patients even existence of the right hypochondrial pain. This was also supported by previous study [23] that compared clinical and pathological characteristics of occult HCV infection patients and untreated chronic HCV patients, but on contrast nearly 50% of NAFLD patients have fatigue and hepatomegaly in agreement with others [24].
The most common laboratory abnormalities often found in NAFLD patients are elevated alanine transaminase (ALT) and aspartate transaminase (AST) which are significantly higher (p value = 0.045 & 0.015 respectively) than that of occult HCV patients confirming the less inflammatory process present in occult HCV provided by the histopsthological examination later and come in context with other reports [23,25,26].
In this study, it was found that the prevalent HCV genotype was type 4a, this is in accordance with the finding that genotype 4 (HCV-4) is the most common variant of the hepatitis C virus (HCV) in the Middle East and Africa, particularly Egypt [27,28].
In this study the insignificant lower necroinflammatory grade (A1) and fibrosis stage (F1) associated with the low number of infected hepatocytes found in occult HCV infection may be related to less liver damage. This goes well with the previous studies [23,25]. While patients with NAFLD/NASH have been noted to have more necroinflammation and fibrosis yet not significant in this study, investigators correlate that to different steps in the Pathophysiology such as predominance of steatosis that is mainly macrovesicular and injury in acinar zone 3; due to zonal localization of DNA damage, products of oxidative damage and expression of CYP 2E142 in zone 3 [29-31].
However, an Egyptian study found that the necroinflammatory activity grade and the fibrosis stage were insignificantly higher in occult HCV infection than in patients without intrahepatic HCV RNA. Furthermore, in European study; out of the 57 patients with occult HCV infection, 20 (35%) had necroinflammatory activity significantly higher than that found in patients without intrahepatic HCV RNA. In addition, the number of cases with fibrosis (F1 according to METAVIR) was significantly higher in patients with occult HCV infection than in patients without intrahepatic HCV RNA [4,20].
In Conclusions: Occult HCV infection must now be accepted as much an entity as occult HBV infection. The absence of serological markers in defining occult HCV infection clearly differentiates this entity from the classical chronic hepatitis C infection. Occult HCV infection is highly prevalent among Egyptian patients with NAFLD. Occult HCV infection seems to induce a mild liver disease. Follow up is recommended for the occult HCV patients to monitor progression to overt disease.
6. ACKNOWLEDGEMENTS
This study was funded by SSI (Sustainable Sciences Institute), California, USA.
REFERENCES
- Berasain, C., Betés, M., Panizo, A., Ruiz, J., Herrero, J.I., Civeira, M.-P. et al. (2000) Pathological and virological findings in patients with aetiology persistent hypertransaminasaemia of unknown. Gut, 47, 429-435. doi:10.1136/gut.47.3.429
- Diodati, G., Pontisso, P., Bonetti, P., Stenico, D., Noventa, F., Alberti, A., et al. (1988) Cryptogenic chronic liver disease and serum or liver hepatitis B virus markers. Their possible correlations and etiologic significance. Digestion, 39, 251-256. doi:10.1159/000199633
- Chemin, I., Zoulim, F., Merle, P., Arkhis, A., Chevallier, M., Kay, A., et al. (2001) High incidence of hepatitis B infections among chronic hepatitis cases of unknown aetiology. Journal of Hepatology, 34, 447-454. doi:10.1016/S0168-8278(00)00100-8
- Castillo, I., Pardo, M., Bartolome, J., Ortiz-Movilla, N., Rodriguez-Inigo, E., de Lucas, S., et al. (2004) Occult hepatitis C virus infection in patients in whom the etiology of persistently abnormal results of liver-function tests is unknown. Journal of Infectious Diseases, 189, 7-14. doi:10.1086/380202
- Abdel-Aziz, F., Habib, M., Mohamed, M.K., Abdel-Hamid, M., Gamil, F., Madkour, S., et al. (2000) Hepatitis C virus (HCV) infection in a community in the Nile Delta: Population description and HCV prevalence. Hepatology, 32, 111-115. doi:10.1053/jhep.2000.8438
- Nafeh, M.A., Medhat, A., Shehata, M., Mikhail, N.N., Swifee, Y., Abdel-Hamid, M., et al. (2000) Hepatitis C in a community in Upper Egypt: I. Cross-sectional survey. American Journal of Tropical Medicine and Hygiene, 63, 236-241.
- El-Sadawy, M., Ragab, H., el-Toukhy, H., el-Mor Ael, L., Mangoud, A.M., Eissa, M.H., et al. (2004) Hepatitis C virus infection at Sharkia Governorate, Egypt: Seroprevalence and associated risk factors. Journal of the Egyptian Society of Parasitology, 34, 367-384.
- El-Zanaty, F. and Way, A. (2009) Egypt demographic and health survey 2008. Ministry of Health, El-Zanaty and Associates and Macro International.
- Mezban, Z.D. and Wakil, A.E. (2006) Hepatitis C in Egypt. American Journal of Gastroenterology.
- Abdel-Hamid, M., Edelman, D.C., Highsmith, W.E., et al. (1997) Optimization, assessment, and proposed use of a direct nested reverse transcribtion-polymease chain reaction protocol for the detection of hepatitis C virus. Journal of Human Virology, 1, 58-65.
- Bedossa, P. and Poynard, T. (1996) An algorithm for the grading of activity in chronic hepatitis C. Hepatology, 24, 289-293. doi:10.1002/hep.510240201
- McOmish, F., Yap, P.L., Dow, B.C., Follett, E.A., Seed, C., Keller, A.J., et al. (1994) Geographical distribution of hepatitis C virus genotypes in blood donors: An international collaborative survey. Journal of Clinical Microbiology, 32, 884-892.
- Constantine, N.T., Abdel-Hamid, M. and Oldach, D.W. (1995) Rapid genotyping of hepatitis C virus. New England Journal of Medicine, 333, 800. doi:10.1056/NEJM199509213331213
- Frank, C., Mohamed, M.K., Strickland, G.T., Lavanchy, D., Arthur, R.R., Magder, L.S., et al. (2000) The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet, 355, 887-891. doi:10.1016/S0140-6736(99)06527-7
- Pybus, O.G., Charleston, M.A., Gupta, S., Rambaut, A., Holmes, E.C. and Harvey, P.E. (2001) The epidemic behaviour of the hepatitis C virus. Science, 292, 2323-2325. doi:10.1126/science.1058321
- Tanaka, Y., Shimoike, T., Ishii, K., Suzuki, R., Suzuki, T., Ushijima, H., et al. (2000) Selective binding of hepatitis C virus core protein to synthetic oligonucleotides corresponding to the 5’ untranslated region of the viral genome. Virology, 270, 229-236. doi:10.1006/viro.2000.0252
- Zakaria, S., Fouad, R., Shaker, O., Zaki, S., Hashem, A., El-Kamary, S.S., et al. (2007) Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clinical Infectious Diseases, 44, 30-36. doi:10.1086/511074
- Schmidt, W.N., Wu, P., Cederna, J., Mitros, F.A., LaBrecque, D.R. and Stapleton, J.T. (1997) Surreptitious hepatitis C virus (HCV) infection detected in the majority of patients with cryptogenic chronic hepatitis and negative HCV antibody tests. Journal of Infectious Diseases, 176, 27-33. doi:10.1086/514033
- Stapleton, J.T., Schmidt, W.N. and Katz, L. (2004) Seronegative hepatitis C virus infection, not just RNA detection. Journal of Infectious Diseases, 190, 651-652. doi:10.1086/421282
- Saleh, A., Yousry, M., Hassan, E.S., et al. (2008) Study of the prevalence of occult Hepatitis C virus infection in patients with persistent hypertransaminasaemia of unknown etiology, and its correlation with hepatic histopathological change.
- Quiroga, J.A., Castillo, I., Bartolome, J., and Carreno, V. (2007) Serum immunoglobulin G antibodies to the GOR autoepitope are present in patients with occult hepatitis C virus (HCV) infection despite lack of HCV-specific antibodies. Clinical and Vaccine Immunology, 14, 1302- 1306. doi:10.1128/CVI.00128-07
- Habib, M., Mohamed, M.K., Abdel Aziz, F., Magder, L.S., Abdel Hamid, M., Gamil, F., et al. (2001) Hepatitis C virus infection in a community in the Nile Delta: Risk factors for seropositivity. Hepatology, 33, 248-253. doi:10.1053/jhep.2001.20797
- Pardo, M., Lo´pez-Alcorocho, J.M., Rodrı´guez-In˜igo, E., Castillo, I., and Carren˜O, V. (2007) Comparative study between occult hepatitis C virus infection and chronic hepatitis C. Journal of Viral Hepatitis, 14, 36-40.
- Sanyal, A.J. (2002) AGA technical review on nonalcoholic fatty liver disease. Gastroenterology, 123, 1705- 1725. doi:10.1053/gast.2002.36572
- Haydon, G.H., Jarvis, L.M., Blair, C.S., Simmonds, P., Harrison, D.J., Simpson, K.J., et al. (1998) Clinical significance of intrahepatic hepatitis C virus levels in patients with chronic HCV infection. Gut, 42, 570-575. doi:10.1136/gut.42.4.570
- Sorbi, D., Boynton, J. and Lindor, K.D. (1999) The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. American Journal of Gastroenterology, 94, 1018-1022. doi:10.1111/j.1572-0241.1999.01006.x
- Kamal, M.S. and Nasser, A.I. (2008) Hepatitis C genotype 4. Hepatology, 47, 1371-1383. doi:10.1002/hep.22127
- Genovese, D., Dettori, S., Argentini, C., Villano, U., Chionne, P., Angelico, M., et al. (2004) Molecular epidemiology of hepatitis C virus genotype 4 isolates in Egypt and analysis of the variability of envelope proteins E1 and E2 in patients with chronic hepatitis. Journal of Clinical Microbiology, 43, 1902-1909. doi:10.1128/JCM.43.4.1902-1909.2005
- Seki, S., Kitada, T., Yamada, T., et al. (2002) In situ detection of lipid peroxidation and oxidative DNA damage in nonalcoholic fatty liver diseases. Journal of Hepatology, 37, 56-62. doi:10.1016/S0168-8278(02)00073-9
- Farrell, G.C. (2003) Nonalcoholic steatohepatitis: What is it, and why is it important in the Asia-Pacific region? Journal of Gastroenterology and Hepatology, 18, 124- 138. doi:10.1046/j.1440-1746.2003.02989.x
- Brunt, E.M. (2007) Pathology of fatty liver disease. Modern Pathology, 20, 40-48. doi:10.1038/modpathol.3800680

