Journal of Encapsulation and Adsorption Sciences
Vol.3 No.1(2013), Article ID:29469,8 pages DOI:10.4236/jeas.2013.31006
Microencapsulation of Essential Oils within Alginate: Formulation and in Vitro Evaluation of Antifungal Activity
1Department of Polymer Materials, Advanced Technology and New Materials Research Institute, City of Scientific Research and Technology Applications, New Borg El-Arab City, Egypt
2Department of Plant Protection, Faculty of Agriculture-Saba Basha, Alexandria University, Alexandria, Egypt
Email: *emadsoliman@mucsat.sci.eg
Received January 21, 2013; revised February 22, 2013; accepted March 1, 2013
Keywords: Essential Oils; Alginate Microspheres; Extrusion; Antifungal Activity
ABSTRACT
Essential oils (EOs) are the volatile lipophilic components extracted from plants. Many EOs have demonstrated strong antimicrobial properties when tested in in vitro experiments. The commercial applications of these EOs require a suitable formulation constituted by biodegradable compounds that protect them from degradation and evaporation at the same time that allows for a sustained release. The objective of this study was therefore to reduce the rate of evaporation of the oil via microencapsulation. Alginate microspheres (AMSs) were prepared using emulsion extrusion method. The AMSs were hardened with a cross-linking agent, calcium chloride. The effects of the three variables: alginate concentration (0.5% - 8%), the amount of cross-linking agent (0.125% - 2%) and time of cross-linking (5 - 30 min.) on loading capacity and encapsulation efficiency (EE, %) were studied. The effect of the amount of cross-linker was significant on loading capacity (%) and EE (%). The AMSs under the optimized conditions provided loading capacity of 22% - 24% and EE of 90% - 94% based on type of EO. The antifungal activity of vapors of microencapsulated and non-microencapsulated oils were evaluated against two of pathogenic fungi species for stored grains: Aspergillus niger and Fusarium verticillioides. The optimized MSs were observed to have a sustained in vitro release profile (50% of the antifungal activity was maintained at the 8th day of the study). In conclusion, encapsulation in Ca-alginate microspheres may effectively reduce the evaporation rate of essential oils, thus increase the potential antifungal activity.
1. Introduction
Nowadays, there is a global concern for the widespread use of pesticides which have significant drawbacks including increased cost, handling hazards, concern about pesticide residues on food, and threat to human health and environment [1]. Public awareness of these risks has increased interest in finding safer pesticides or alternative food protectants to replace synthetic chemical pesticides. Therefore, the interest in natural products having an antimicrobial activity to preserve food quantity and quality has increased, because they tend to have low mammalian toxicity, less environmental effects and wide public acceptance [2,3].
Many essential oils (EOs), such as garlic, cinnamon, thyme, oregano, clove, basil, coriander, citrus peel, eucalyptus, ginger, rosemary, and peppermint, among others, have been demonstrated antimicrobial activity.
A significant body of research on EOs concerning in vitro evaluation has been conducted on the liquid phase [2]. However, the biological activity of EOs can be lost by volatilization of active components or its degradation by act of high temperatures, oxidation and UV light [4]. These disadvantages make the commercial application of these oils limited. On the other side, EOs in the vapor phase have the potential for use as fumigants [5]. Therefore, formulation of essential oils involves their preparation in liquid forms (emulsions, micelles, liquid solutions etc.), semi-liquid forms (gels, liposome, etc.) or solid forms (microcapsules or microspheres) have to be employed for controlling release of active ingredients and protecting them from the external environment. Microencapsulation as one of the most efficient methods of formulation by which solids, liquids or even gases have the desired characteristics in extremely tiny amounts may be enclosed in microscopic particles formation of thin coatings of wall material with limited permeability around the substances [6]. There are many researches on microencapsulation of EOs using several methods including spray-drying [7], simple coacervation [8], complex coacervation [9] emulsion extrusion [10] and supercritical fluid precipitation [11]. Emulsion extrusion is considered as the most common approach of microencapsulation and might be achieved by emulsifying or dispersing the hydrophobic components in an aqueous solution where gelation occur (ionotropic or thermal) [10].
By using emulsion extrusion for microencapsulation, a broad selection of polymer coatings (“shell”) and methods of deposition are available, which are easily adaptable to large-scale production. Alginates are natural commonly used as wall materials since it shows high toughness and it has considerable effects on the mechanical stability of beads. Chemically, alginates are naturally occurring polycarbohydrates consisting of copolymers of α-L-glucuronic acid (G) and β-D-mannuronic acid (M). The relative amounts of these two building blocks influence the total chemistry of this biopolymer, where G/M ratio determines the permeability properties of the swollen alginate gel which envelops the essential oils [12].
The main aim of the present study is to develop microencapsulation of clove, thyme and cinnamon oils in calcium alginate was done by employing the emulsion extrusion technology in order to achieve their optimal antifungal activity against two of pathogenic fungi species for stored grains: Aspergillus niger and Fusarium verticillioides. Various operational parameters such as concentration of alginate and cross-linking agent and time of cross-linking on loading capacity and encapsulation efficiency (EE, %) were studied. Furthermore, this experimental study provides an insight for relating the antifungal efficiency of microencapsulated essential oils with storage time.
2. Materials and Methods
2.1. Materials
Three types of essential oils for medical use were purchased from the local market which are extracted from Clove (Eugenia caryophyllata), thyme (Thymus vulgaris) and Cinnamon (Cinnamomum zeylanicum), sodium alginate (low viscosity) and n-hexane were purchased from Sigma-Aldrich, (Germany). Calcium chloride (CaCl2) was purchased from Park Scientific limited (UK). Sodium citrate was obtained from Pratap chemical industries (India). All other reagents were analytical grade and used without further purification.
2.2. Fungi Species
Two species of stored grains pathogenic fungi, Aspergillus niger and Fusarium verticillioides were obtained from Genetic Engineering and Biotechnology Research Institute (CSRTA). The culture of each fungus were maintained on Czapeks-Dox agar and stored at ≤4˚C.
2.3. Encapsulation of EOs
Micro-encapsulation of oil was conducted using emulsion extrusion technique described by Chan [13]. Where, sodium alginate was dissolved in distilled water to produce alginate solutions with concentration of 0.5, 1, 2, 4 or 8 w/v%, the solutions were left standing for 24 h to disengage bubble before use. Afterwards, sodium alginate solution (30 g) and EO (10 g) were homogenized into a 200 mL beaker with stirring at a speed of 300 rpm for 45 min by a magnetic stirrer. The oil was gradually added to the alginate solution during mixing until the desired oil loading was obtained. Fifty milliliters of alginate-oil emulsion was then sprayed into a collecting water bath containing calcium chloride solution (0.125, 0.25, 0.5, 1, 2 w/v%) using an Inotech Encapsulator IER-50 (Switzerland) with a 500-µm nozzle [14]. This operation was adjusted at a frequency of 550 Hz and a voltage of 1.40 kV. The resulting microcapsules were allowed to harden in the CaCl2 solution for 5, 10, 15, 20, 25 or 30 min. The oil-loaded alginate beads were collected from the cross-linking solution using a sieve. Finally, the microbeads were rinsed twice with distilled water, tissue paper was used to absorb the surface excessive water and oil onto the wet microcapsules. Quantification of EOs loaded within AMSs was conducted by using the method described by Parris and his colleagues [15] with a slight modification, where the amount of EO enveloped into the microbeads was quantified by extracting the loaded oil from 0.5 g of these beads via their dissolution with 5 mL of sodium citrate (0.055 M) and 5 mL n-hexane. The absorbance was then measured at wavelength of 275 nm for thyme oil and at 280 nm for clove and cinnamon oils by using spectrophotometer model Ultrospec 2000 (Pharmacia Biotech Co. Cambridge, England). The amounts of EOs were calculated from plotted the standard curves for EOs with usage of dissolved alginate microbeads with no EO as a control.
Loading capacity (%) was calculated from the following equation:
 (1)
(1)
where Wo = Quantity of loaded EOWMS = Quantity of MSs.
Encapsulation efficiency (EE, %) was calculated from the following equation:
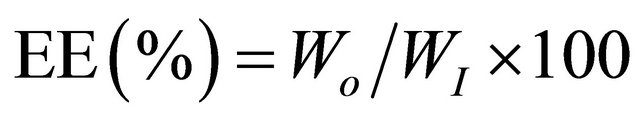 (2)
(2)
where Wo = Quantity of loaded EOWI = Initial quantity of EO.
For each formulation, the loading capacity and encapsulation efficiency were determined for triplicate, the average was calculated.
2.4. Scanning Electron Microscopic Analysis of MSs
The EOs microspheres were observed visually and analyzed using high-resolution scanning electron microscope (SEM) with suitable accelerating voltage and magnifications. The SEM analysis was carried out at Central Laboratory of Materials Characterization of Advanced Technology and New Materials Research Institute (ATNMRI), City of Scientific Research and Technology Applications (SRTA) (Egypt) using JOEL 6360LA scanning electron microscope (JEOL Ltd., Tokyo, Japan) operated at an acceleration voltage of 15 kV. Fine coat-ion sputter device, JFC1100E (JOEL Ltd., Tokyo, Japan), consisting of basic and rotary pump unit was used to perform efficient and rapid metal coating. The coating pressure was 0.17 torr at 1200 V and 8 mA. The scanning electron micrographs were used for elucidating the structural characteristics of alginate microspheres.
2.5. Assessment of Antifungal Activity of Vapors of Encapsulated EOs
Antifungal activity was assessed by the method described by Feng and others [16] with a slight modification, these biological assays were conducted using Petri plates (diameter 90 mm) of Czapeks-Dox agar medium (15 ml/ plate) with making well (diameter 10 mm) in the center of Petri plates using a flamed cork borer and inoculated with 100 µL suspension of the tested fungi from oneweek old culture (A disc with diameter of 3 cm of each fungus was cut from periphery and suspended into 10 mL of sterile distilled water. Then the resulting was vigorously shaken). A sterilized filter paper disc with diameter of 30 mm was fixed in the upper lid of the Petri dish. Varying volumes of EO (1, 2.5, 5 and 10 µl was placed in each plate either in free or micro-encapsulated form. Where, definite volume of free EO was distributed onto filter paper (at the center). However, the certain amounts of MSs were adhered using one milliliter of solidification sterilized agar that was first placed at the center of the upper lid of the Petri dish. So the volume of EO enveloped into each amount of MSs was equivalent to volumes 1, 2.5, 5 and 10 µl of EOs. Blank filter paper disc served as a control for the free oils while for the encapsulated oils the control was alginate microbeads (with no EOs). The surfaces of oil-loaded filter paper discs or adhered MSs ware at a distance of about 6 mm from the medium surface (the growth surface of the tested fungi). Plates were tightly sealed with an adhesive tape and incubated at 27˚C ± 2˚C for 5 days. The growth inhibition was determined by measuring the diameter of fungal growth zones. Three replicates were used per each test and percentage of growth inhibition was calculated by the following equation [17]
 (3)
(3)
where Dc = diameter of fungal colony in control setDt = diameter of fungal colony in treatment set.
2.6. Antifungal Activity Profile of Vapors of Encapsulated EOs
To assess the antifungal efficiency of free and encapaculated oils during storage, the oil distributed onto filter paper disc (free oils) and encapsulated oil (MSs) were stored at 28˚C ± 2˚C for 2, 4 and 8 days. Afterwards, the biological assay was done for the stored samples as described above by using dosage of 5 µl/plate against A. niger.
2.7. Statistical Analysis
Data of the present study were subjected to the analysis of variance test (ANOVA) as complete randomized design. The least significant differences (LSD) at the 5% level of probability were determined using a computer program [18].
3. Results and Discussion
3.1. Operational Parameter Affecting Loading Capacity and Encapsulation Efficiency
Influence of various operational parameters of microencapsulation process on the loading capacity (%) and encapsulation efficiency (EE, %) including alginate concentration, cross-linking agent concentration and time were investigated.
3.2. Alginate Concentration
To explore the effect of alginate concentration on the loading capacity of the obtained beads, different concentrations of alginate (0.5% - 8%) were tried with keeping the concentration of calcium chloride constant at 0.5% and cross-linking time of 20 min. The results presented in Figure 1 which indicated that the loading capacity increased with increasing the alginate concentration from 0.5% to 2%, where it reached about 23% for thyme, clove and cinnamon oil. Whereas, further increase in alginate concentration led to decrease the loading capacity to ~5% with the different oils at alginate concentration of 8%. This increase in the loading capacity with increasing the alginate concentration can be attributed to that increasing of alginate concentration leads to forming

Figure 1. Effect of alginate concentration on the loading capacity and the encapsulation efficiency.
a dense network structure with cohesive vacancies (pores) that entrap the essential oils droplets (proper pores size with homogeneous distribution). These results are in agreement with those reported by Manjanna and his co-workers who found that the encapsulation efficiency increased with increasing concentration of the sodium alginate [19]. While, the decline in the loading capacity with increasing the alginate concentration over 2% can be explained on the basis that this increases the space occupied by alginate causing a decrease of the free volume within the polymer matrix (a compact structure with smaller pores sizes), and subsequently the amount of oil that can be entrapped within these pores will be decreased. This assumption was supported by findings of Sevda and Rodrigues that indicated that higher alginate concentration leads to a decrease of the pores sizes forming in the resultant microbeads [20].
3.3. Calcium Chloride Concentration
Cross-linking agent concentration as an affecting factor on the loading capacity was studied by using varying concentrations of calcium chloride (0.125% - 2%) with maintaining alginate concentration at 2% and crosslinking time of 20 min. The results of this study were presented in Figure 2 indicating that the increase of calcium chloride concentration from 0.125% to 0.5% leads to increase the loading capacity of the resultant microbeads to reach ~23% for thyme, clove and cinnamon oil. These results are in agreement with those obtained by Manjanna and his colleagues who found that by increasing calcium chloride concentration from 1% - 5%, the drug entrapment efficiency were found to increase from ~83% to ~93% [19]. Nevertheless, increasing concentration of the calcium chloride over 0.5% led to a gradual decrease in the loading capacity to reach about ~18%, 12%, and ~15% for thyme, clove and cinnamon oil, respectively at calcium chloride concentration of 2%. It is obvious that increasing calcium chloride concentration certainly leads to introduce higher levels of Ca2+ ions

Figure 2. Effect of calcium chloride concentration on the loading capacity and the encapsulation efficiency.
causing cross-linking between the alginate chains and consequently forms a dense cohesive structure resulting in more capacity for the resultant beads to entrap higher amount of oil this supported also by findings of Manjanna and others [19]. While, decrease of the loading capacity of the obtained micro-beads with increasing the calcium chloride concentration over 2% can be explained on the basis of the higher approaching of the alginate chains and subsequently decreasing the pores sizes forming within alginate matrices resulting for squeezing the gel micro-beads leading to decrease of the loading capacity.
3.4. Cross-Linking Time
The cross-linking time is also another parameter of cross-linking process affecting the loading capacity of oil-encapsulated micro-beads. Therefore, the encapsulation process was done using alginate concentration of 2% and concentration of calcium chloride of 0.5%, whereas time of the cross-linking process was changed from 5 to 30 min. Results of these experiments shown in Figure 3 which indicated that extending cross-linking time from 5 to 20 minutes leads to increase the loading capacity to ~23%, ~24% and 22% for thyme, clove and cinnamon oil, respectively. Notwithstanding, extending the time of cross-linking over 20 min. leads to decrease the loading capacity till reach ~18%, ~20% and ~14% for thyme, clove and cinnamon oil, respectively with cross-linking time of 30 min. These results can be elucidated on the basis of gelling (alginate cross-linking) performance, the cohesion of the formed gel and partition of the oil between the two environments (within the micro-beads and aqueous medium) that consequently affect entrapping the oil within the polymer matrices or its releasing outside the beads. Where, the released amount of oil is more pronounced when the time of gel formation extended [21]. These results supported by findings of Lai and his co-workers who found that the essential oil encapsulation efficiency decreased when the cross-linking time in-

Figure 3. Effect of cross-linking time on the loading capacity and the encapsulation efficiency.
creased [22]. Moreover, Kulkarni and his colleagues found that the percentage of entrapment efficiency decreased with increase in the time of exposure to the cross-linking agent from 10 to 30 min. by about 15% [23]. This behavior is probably due to a partitioning of the oil components into the aqueous phase by increasing the contact.
From the previous results, the best formulation was: Sodium alginate concentration: 2% w/v, calcium chloride concentration: 0.5% (w/v) and cross-linking time: 20 min. Which gave maximum loading capacity (thyme oil: ~23.4%, clove oil ~23.5% and cinnamon oil ~22.5%) and maximum encapsulation efficiency (thyme oil: ~94%, clove oil ~94% and cinnamon oil ~90%).
3.5. Microstructure of AMSs
Topographical features and microstructure of plain alginate microspheres (non-loaded with EO) or that having EO-loaded highest loading capacity (best formulation prepared at the optimum conditions) were shown in Figure 4. The micrograph of plain alginate microspheres (non-loaded with EO) (Figure 4(A)) exhibited that the surface of these spheres had smooth textural characteristics (high compressive strength, non-shown data). However, EO-loaded Ca-alginate microspheres (Figure 4(B)) exhibited crimpy surface with appearance of noticeable lumps. This can be explained on the basis of the deposition of EO droplets onto the outer or inner surface of the external layer of alginate microbeads, where the presence of oil components onto alginate surfaces can lead to plasticize their structure and forming these lumps. On the other side, cross-section of EO loaded Ca-alginate microspheres (Figure 4(C)) indicated that the structure of these beads appear as a three-dimensional, porous sponge. This cellular structure can be clearly shown in Figure 4(D) that exhibited cavity of the cell and its wall. Furthermore, the wrinkling of cell wall appeared obviously. This egg-box structure confirms the entrapment of EOs into the cells of alginate matrix.
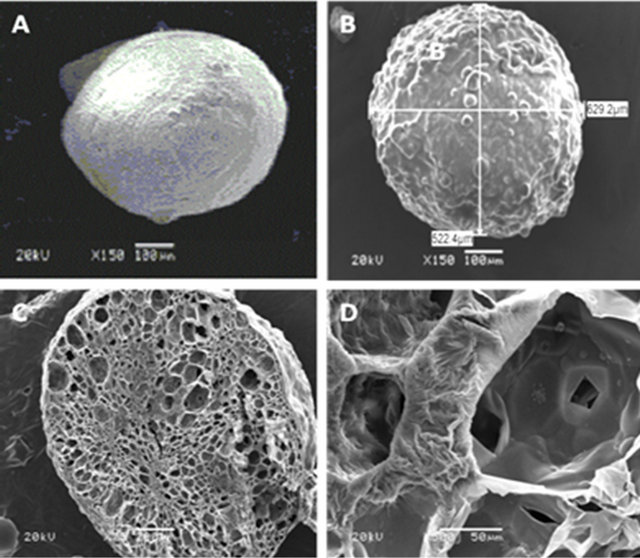
Figure 4. SEM graphs of alginate microspheres, plain (A), EO-loaded (B), cross-section of EO-loaded MS (C) and its microstructure (D).
3.6. Antifungal Activity of Vapors of Encapsulated EOs
The antifungal activity of the vapors of tested EOs (cinnamon, clove and thyme) was evaluated with radial growth technique at several dosages (1, 2.5, 5 and 10 µl/ plate) against two species of grains pathogenic fungi.
3.6.1. Aspergillus Niger
Data in Figure 5 clarified that cinnamon oil was the superior oil which; recorded 100% growth inhibition starting at 2.5 µl against A. niger comparing with clove and thyme oils which recorded 83% and 71% growth inhibition with 2.5 µl receptively, while they showed entire inhibition beginning from dosage of 5 µl. These findings are in agreement with the results reported by Guynot and others who reported that Aspergillus niger was totally inhibited by vapor of pure cinnamon, clove and thyme essential oils at dosage of 50 µl [24]. However, Paster and his colleagues showed that thyme essential oil vapor produced a significantly reduction in colony diameter of A. niger at 3 µl/L [2]. Whereas, the results showed that the oils beads had a strong inhibition effect against A. niger causing complete inhibition begging from 5 µl/ plate. While the beads of cinnamon, clove and thyme oils gave growth inhibition 92%, 87% and 79% at the dosage of 5 µl, respectively.
3.6.2. Fusarium Verticillioides
Results shown in Figure 6 revealed that cinnamon, thyme and clove oils had higher antifungal activity against F. verticillioides. Where, cinnamon oil was the most effective one by giving 100% growth inhibition at 1 µl/plate. Thyme oil showed also a high efficiency toward

Figure 5. Antifungal activity of vapors of the free and encapsulated essential oils against A. niger.

Figure 6. Antifungal activity of vapors of the free and encapsulated essential oils against F. verticillioides.
this fungus by achieving 100% growth inhibition at dose of 5 µl/plate. However, clove oil gave 100% growth inhibition at dosage of 10 µl/plate. Soliman and Badeaa found that the thyme and cinnamon oils had high antifungal activity against Fusarium verticillioides [25]. Whilst, the antifungal activity results of encapsulated oils indicated that cinnamon oil-loaded alginate beads have the highest activity. Where, they gave 100% growth inhibition for this fungus at the lowest dosage (1 µl/plate) as the free oil. Moreover, the activity of thyme oil beads were more pronounced than these for clove oil, where they showed antifungal activity of 65% and 39% respectively, at the lowest applied dosage.
By comparing the antifungal activity of vapors of both of free and loaded oils as shown in Figures 5 and 6, no significant difference has been observed at comparable dosage. This indicated that the efficiency of oils-loaded in alginate matrix did not affect by the encapsulation process. These results are in agreement with those obtained by Pandey and his co-workers who found that formulation of different polymers gel containing cardamom, coriander and cinnamon essential oils does not decrease their antimicrobial activity [26]. Also, Leimann and others found that the encapsulation process of lemongrass essential oil did not cause any deterioration in the essential oil, and their results indicated that there were no significant alterations in the EO during the microencapsulation and also that the biological activity of the essential oil was not affected by the microencapsulation process [27]. On the other side, to explore the capability for both of free and encapsulated oils to maintain their antifungal activity through storage, the activity profile of free and loaded oils were detected.
3.7. Antifungal Activity Profile of Encapsulated EOs
The activity profile of free and encapsulated tested oils was taken part against A. niger using dosage of 5 µl/plate which gave fully growth inhibition with the encapsulated and free tested oils after different times (0, 2, 4, 8 days). The results were shown in Figure 7. They exhibited that the antifungal activity of free oils was decreased by about 90% after storage periods of 2 days. Whereas, after storage of 8 days as shown in Figure 7, the antifungal activity of these oils became so weak or almost nil, where cinnamon oil didn’t exhibit any activity. On the other side, the encapsulated clove and thyme oils still maintain around half of their antifungal activity even after 8 days of storage. Whereas, this activity was reduced to 29% in case of cinnamon oil. These results are in agreement with findings of Passino and others [28]. These results can be attributed to volatilize the most of volatile components responsible for the antimicrobial characteristics of these oils. Normally, this effect was more pronounced for the free oils comparing with these encapsulated in alginate matrix. Whereas, the difference between the antifungal activities of these three oils after storage depends on the volatilization extent of their active components.
4. Conclusion
Based on the pervious results, it can be indicated that the encapsulation process of tested oils can be considered as inexpensive and efficient technique to maintain the antifungal activity of these oils and promote the ease of han-

Figure 7. Antifungal activity profile of free and encapsulated oils against A. niger.
dling of the oils, where these microencapsulated oils, thyme, clove and cinnamon exhibited high growth inhibition for Aspergillus niger and Fusarium verticillioides. Moreover, these EO-loaded Ca-alginate microspheres can maintain 30% - 50% of the antifungal activity of their enveloped oils after storage time of 8 days based on the type of these oils, whilst all types of these free EOs lost their entire antifungal activity after two days of storage. The results obtained from this study suggest that of all formulation techniques, microencapsulation seems to be the best choice for increasing the use of EOs. The microencapsulation in alginate is expected to be suitable for other hydrophobic essential oils.
REFERENCES
- N. Paster and L. B. Bullerman, “Mould Spoilage and Mycotoxin Formation in Grains as Controlled by Physical Means,” International Journal of Food Microbiology, Vol. 7, No. 3, 1988, pp. 257-265. doi:10.1016/0168-1605(88)90044-X
- N. Paster, M. Menasherov, U. Ravid and B. Juven, “Antifungal Activity of Oregano and Thyme Essential Oils Applied as Fumigants against Fungi Attacking Stored Grain,” Journal of Food Protection, Vol. 58, No. 1, 1995, pp. 81-85.
- T. R. Hamilton-Kemp, D. D. Archbold, J. H. Loughrin, R. A. Andersen, C. T. McCracken, R. W. Collins and E. Fallik, “Stimulation and Inhibition of Fungal Pathogens of Plants by Natural Volatile Phytochamicals and Their Analogs,” Current Topics in Photochemistry, Vol. 4, 2000, pp. 95-104.
- J. F. Ayala-Zavala, L. del Toro-Sanchez, E. AlvarezParrilla and G. A. Gonzalez-Aguilar, “High Relative Humidity In-Package of Fresh-Cut Fruits and Vegetables: Advantage or Disadvantage Considering Microbiological Problems and Antimicrobial Delivering Systems,” Journal of Food Science, Vol. 73, No. 4, 2008, pp. 41-47. doi:10.1111/j.1750-3841.2008.00705.x
- T. P. Bergkvist, “Antimicrobial Activity of Four Volatile Essential Oils,” M.Sc. Thesis, School of Biomedical Science, Charles Sturt University, Dubbo, 2007.
- H. Yoshizawa, “Trends in Microencapsulation Research,” Kona, Vol. 22, 2004, pp. 22-31.
- J. Shaikh, R. Bhosale and R. Singhal, “Microencapsulation of Black Pepper Oleoresin,” Food Chemistry, Vol. 94, No. 1, 2006, pp.105-110. doi:10.1016/j.foodchem.2004.10.056
- J. Lazko, Y. Popineau and J. Legrand, “Soy Glycinin Microcapsules by Simple Coacervation Method,” Colloids and Surfaces B: Biointerfaces, Vol. 37, No. 1, 2004, pp. 1-8. doi:10.1016/j.colsurfb.2004.06.004
- C. P. Chang, T. K. Leung, S. M. Linc and C. C. Hsu, “Release Properties on Gelatin-Gum Arabic Microcapsules Containing Camphor Oil with Added Polystyrene,” Colloids and Surfaces B: Biointerfaces, Vol. 50, No. 2, 2006, pp. 136-140. doi:10.1016/j.colsurfb.2006.04.008
- S. Yuliani, P. J. Torley, B. D'Arcy, T. Nicholson and B. Bhandari, “Extrusion of Mixtures of Starch and DLimonene Encapsulated with β-Cyclodextrin: Flavour Retention and Physical Properties,” Food Research International, Vol. 39, No. 3, 2006, pp. 318-331. doi:10.1016/j.foodres.2005.08.005
- Á. Martín, S. Varona, A. Navarrete and M. J. Cocero, “Encapsulation and Co-Precipitation Processes with Supercritical Fluids: Applications with Essential Oils,” Open Chemical Engineering, Vol. 4, 2010, pp. 31-41. doi:10.2174/1874123101004020031
- B. Amsden, “Solute Diffusion within Hydrogels,” Macromolecules, Vol. 31, No. 23, 1998, pp. 8382-8395. doi:10.1021/ma980765f
- E. S. Chan, “Preparation of Ca-Alginate Beads Containing High Oil Content: Influence of Process Variables on Encapsulation Efficiency and Bead Properties,” Carbohydrate Polymers, Vol. 84, No. 4, 2011, pp. 1267-1275. doi:10.1016/j.carbpol.2011.01.015
- Q. Wang, J. Gong, X. Huang, H. Yu and F. Xue, “In Vitro Evaluation of the Activity of Microencapsulated Carvacrol against Escherichia coli with K88 Pili,” Applied Microbiology, Vol. 107, 2009, pp. 1781-1788. doi:10.1111/j.1365-2672.2009.04374.x
- N. Parris, P. H. Cooke and K. B. Hicks, “Encapsulation of Essential Oils in Zein Nanospherical Particles,” Journal of Agriculture and Food Chemistry, Vol. 53, No. 12, 2005, pp. 4788-4792. doi:10.1021/jf040492p
- W. Feng, J. Chen, X. Zheng and Q. Liu, “Thyme Oil to Control Alternaria alternata in Vitro and in Vivo as Fumigant and Contact Treatments,” Food Control, Vol. 22, No. 1, 2011, pp. 78-81. doi:10.1016/j.foodcont.2010.05.010
- D. K. Pandey, H. Chandra and N. N. Tripathi, “Volatile Fungitoxic Activity in Higher Plants with Special Reference to that of Callistemon lanceolatus D.C.,” Phytopathologische Zeitschrift, Vol. 105, No. 2, 1982, pp. 175- 182. doi:10.1111/j.1439-0434.1982.tb00675.x
- Costat Software, “Microcomputer Program Analysis,” CoHort Software, Berkely, 1988.
- K. M. Manjanna, B. Shivakumar and T. M. P. kumar, “Formulation of Oral Sustained Release Aceclofenac Sodium Microbeads,” International Journal of Pharm Tech Research, Vol. 1, No. 3, 2009, pp. 940-952.
- S. B. Sevda and L. Rodrigues, “The Making of Pomegranate Wine Using Yeast Immobilized on Sodium Alginate,” African Journal of Food Science, Vol. 5, No. 5, 2011, pp. 299-304.
- A. A. El-Zatahry, E. A. Soliman, E. A. Hassan and M. S. M. Eldin, “Preparation and in Vitro Release of Theophylline Loaded Sodium Alginate Microspheres,” Proceedings of the ASTF, Scientific Research Outlook Conference, No. 155, 2006, pp. 1-20.
- F. Lai, G. Loy, M. Manconi, M. L. Manca and A. M. Fadda, “Artemisia arborescens L. Essential Oil Loaded Beads: Preparation and Characterization,” AAPS PharmSciTech, Vol. 8, No. 3.2007, pp. 1-7.
- A. R. Kulkarni, K. S. Soppimath, T. M. Aminabhavi, A. M. Dave and M. H. Mehta, “Glutaraldehyde Crosslinked Sodium Alginate Beads Containing Liquid Pesticide for Soil Application,” Journal of Controlled Release, Vol. 63, No. 1-2, 2000, pp. 97-105. doi:10.1016/S0168-3659(99)00176-5
- M. E. Guynot, A. J. Ramos, L. Setó, P. Purroy, V. Sanchis and S. Marín, “Antifungal Activity of Volatile Compounds Generated by Essential Oils against Fungi Commonly Causing Deterioration of Bakery Products,” Applied Microbiology, Vol. 94, No. 5, 2003, pp. 893-899. doi:10.1046/j.1365-2672.2003.01927.x
- K. M. Soliman and R. I. Badeaa, “Effect of Oil Extracted from Some Medicinal Plants on Different Mycotoxigenic Fungi,” Food and Chemical Toxicology, Vol. 40, No. 11, 2002, pp. 1669-1675. doi:10.1016/S0278-6915(02)00120-5
- A. Pandey, J. V. Jagtap and S. A. Polshettiwar, “Formulation and Evaluation of in Vitro Antimicrobial Activity of Gel Containing Essential Oils and Effect of Polymer on their Antimicrobial Activity,” International Journal of Pharmacy and Pharmaceutical Sciences, Vol. 3, No. 1, 2011, pp. 234237.
- F. V. Leimann, O. H. Gonçalves, R. A. F. Machado and A. Bolzan, “Antimicrobial Activity of Microencapsulated Lemongrass Essential Oil and the Effect of Experimental Parameters on Microcapsules Size and Morphology,” Materials Science and Engineering: C, Vol. 29, No. 2, 2009, pp. 430-436. doi:10.1016/j.msec.2008.08.025
- S. G. Passino, E. Bazzoni and M. D. L. Moretti, “Microencapsulated Essential Oils Active against Indian Meal Moth,” Boletín de Sanidad Vegetal Plagas, Vol. 30, 2004, pp. 125-132.
NOTES
*Corresponding author.

