International Journal of Organic Chemistry
Vol.09 No.01(2019), Article ID:91192,6 pages
10.4236/ijoc.2019.91006
Efficient Cross-Coupling Reaction of Aryltrifluoroborates and Aroyl Chlorides for the Synthesis of Fluorine Substituted Aromatic Ketones
Mohammed Al-Masum*, Tasfia Islam, Grady Clopton
Department of Chemistry, Tennessee State University, Nashville, TN, USA

Copyright © 2019 by author(s) and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 26, 2019; Accepted: March 15, 2019; Published: March 18, 2019
ABSTRACT
The direct aroylation of ArCOPdCl with potassium aryltrifluoroborates establishes a new cross-coupling synthetic tool for the synthesis of various fluorine substituted benzophenones. The new microwave irradiated process is very efficient and produce high yield benzophenone products within minutes.
Keywords:
ArBF3K, Aroyl Chlorides, Direct Aroylation to Ketones, Minute Reaction

1. Introduction
The selective functionalization of potassium aryltrifluoroborates into fluoro-substituted aromatic ketones is an important synthetic task. Fluoro-substituted aromatic ketones are very active pharmaceutical ingredients useful for pharmaceutical application such as anti-inflammatory effects. Introducing fluorine moiety is demanding methodology in medicinal chemistry to adjust binding affinity of small molecules for biological targets and control metabolic reactivity [1] [2] . Fluorinated benzophenones served as starting point for the synthesis of many small benzophenone analogs. Transition metal catalyst, especially palladium, is very popular in organic synthesis because it has a unique ability to activate various organic compounds. This activation can catalyze the formation of new bonds. J. K. Stille first successfully developed the direct cross-coupling reaction of organotin compounds and benzoyl chlorides for benzophenone synthesis [3] [4] . Haddach also has reported a similar cross-coupling reaction by using arylboronic acids [5] [6] . In this work, a new reaction method establishes for the direct aroylation reaction of potassium aryltrifluoroborates and ArCOCl in the presence of PdCl2(Ph3P)2 (Scheme 1).
2. Results and Discussion
This direct aroylation reaction combines PdCl2(Ph3P)2 with K2CO3, as a new catalyst system along with potassium aryltrifluoroborates and fluoro substituted aroyl chlorides in a dry microwave vial under argon. The resulting mixture microwave in 1,4-dioxane at 150˚C for 20 min and obtained high yield products of various fluoro substituted benzophenones. Recently, new synthetic methods for crotonophenones by cross coupling of potassium allyl trifluoroborate with aroyl chlorides, and chalcones from potassium styryl trifluoroborates and aroyl chlorides have been established [7] [8] . In both cases, PdCl2 (dtbpf) complex has shown remarkable catalyst effect for successful transformations (Scheme 2). In line with those findings, it has assumed that same catalyst system would work for cross coupling of aryl trifluoroborates with aroyl chlorides. Surprisingly, no desired benzophenones observed as major products.
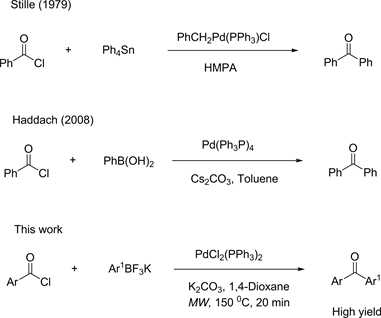
Scheme 1. Aryltrifluoroborates for aroylation reactions.
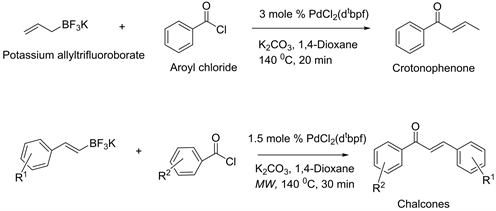
Scheme 2. Organotrifluorborates and direct aroylation to crotonophenones and chalcones.
After further investigation with several palladium complexes and bases to find a suitable conditions for aroylation of potassium aryltrifluoroborates under microwave irradiation, the effective catalyst effect of PdCl2(Ph3P)2 complex has been observed and successfully synthesize series of fluoro-substituted aromatic ketones in high yields.
The results summarize in Figure 1. It appears that 1,4-dioxane as the solvent system gave successful results in cross-coupling reaction. The biggest challenge of this project was the formation of homo-coupling product instead of cross coupling. Often times the ArBF3K reacts with itself giving biphenyl as homo coupling product that observed in GC-MS. This problem is mostly overcome by applying 3 mole % PdCl2 (Ph3P)2 as catalyst.
The mechanism for the direct cross-coupling reaction of potassium organotrifluoroborates and benzoyl chlorides proposes in Scheme 3. The catalytic cycle involves the palladium insertion by oxidative addition followed by ligand exchange with the K2CO3. Then occurring transmetallation of organoboron species to the organopalladium, followed by forming the desired cross coupling product by reductive elimination.
Figure 1. Cross-coupling of AryIBF3K and ArCOPdCl for Fluorinatedketoonesa. aIsolated products separated by silica gel chromatography.
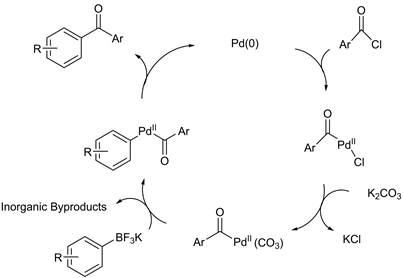
Scheme 3. Catalytic cycle for direct aroylation of ArylBF3K.
3. Conclusions
This work demonstrates the novel application of potassium aryltrifluoroborates with aroyl chlorides for cross coupling reaction. A new catalyst system is established. The application of microwave heating drastically reduced the amount of time to 20 minutes. Usually this type of coupling requires 8 - 24 hours heating in conventional heating system. The direct aroylation of potassium aryltrifluoroborates is great addition to the direct aroylation of potassium styryltrifluoroborates, potassium allyltrifluoroborate, and potassium crotyltrifluoroborate.
Experimental
Synthesis of aryl trifluoroborates (BF3K); the starting material
ArBF3K is synthesized from boronic acid. In a typical synthesis, in a round bottom flask with a stirrer, about 20 mL of methanol was added to 20 mmol of boronic acid. This mixture was completely dissolved in the methanol. The solution was clear. On a separate small beaker, 120 mmol (about 9.36 g) potassium hydrogen fluoride (KHF2) was dissolved in 20 mL of DI water. This KHF2 solution then added to the methanol mixture while the mixture was stirring vigorously. This resulting thick mixture was on vigorous starring for 4 hours. The solvent in the mixture dried up by using rotary evaporator followed by high vacuo for complete dry. To remove excess KHF2 from the crude product, 250 mL of boiled acetone added into the mixture. The clear liquid part of the mixture then filtered through sintered funnel and the filtrate evaporated in a rotary evaporator until it was completely dry. To recrystallize the mixture, the product was dissolved in minimum amount of acetonitrile (CH3CN) followed by adding diethyl ether until solid crystal formed. The crystallized solid potassium aryltrifluoroborate product was collected in high yields.
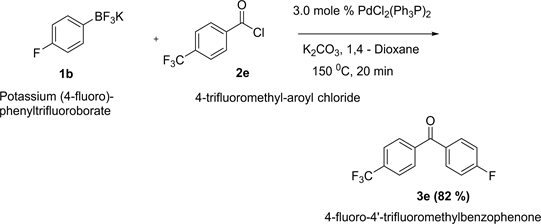
Synthesis of 4-fluoro-4’-trifluoromethylbenzophenone is arepresentative one.
A small dry microwave reaction vial loaded with 0.5 mmol of potassium (4-trifluoromethyl)phenyltrifluoroborate, 2 mmol of K2CO3 and 0.015 mmol of PdCl2(Ph3P)2 (11.0 mg). The vial was capped with air-tight septum followed by flushing with argon. The palladium is very sensitive to air. It was made sure that this catalyst was added at the end to prevent any air exposer. 121.0 µL of 4-fluoroaroyl chloride (1.0 mmol) was transferred to the mixture via syringe. After adding 5.0 mL of 1,4-Dioxane as solvent, the resulting mixture was stirred at room temperature for couple of minutes and then inserted the vial into microwave system and heated at 150˚C for 20 min. The crude product was filtered through celite pad by using ethyl acetate. After examining the GC-MS, 10 g of silica gel was added to the filtrate and evaporated the solvent by rotary evaporator. The compound adsorbed in silica gel was then transferred into silca gel column for chromatography.
Data for the products shown in Figure 1
Compound 3a, 1HNMR (CDCl3, 400 MHz) δ 8.31 - 7.19 (m, 8H); 13CNMR (CDCl3, 100 MHz) δ 193.2, 142.3, 134.4, 132.1, 130.6, 123.7, 120.4; 19FNMR (CDCl3, 400 MHz) δ −57.7.
Compound 3b, 1HNMR (Acetone-d6, 400 MHz) δ 8.12 - 7.07 (m, 8H), 3.91 (s, 3H); 13CNMR (Acetone-d6, 100 MHz) δ 193.9, 167.3, 165. 8, 164.4, 163. 1, 138. 1, 131.1, 123.7, 121.4, 1114.5, 56.0; 19FNMR (Acetone-d6, 400 MHz) δ −58.5.
Compound 3c, 1HNMR (CDCl3, 400 MHz) δ 7.78 - 7.07 (m, 8 H)13CNMR (CDCl3, 100 MHz) δ 193.7, 167.2, 163.8, 152.1, 135.7, 133.2, 131.7, 120.8, 116.1, 19FNMR (CDCl3, 400 MHz) δ −57.6, 105.2.
Compound 3d, 1HNMR (CDCl3, 400 MHz) δ 7.87 - 7.33 (m, 8 H) 13CNMR (CDCl3, 100 MHz) δ 193.6, 152.3, 135.3, 131.9, 120.3, 29.7, 19FNMR (CDCl3, 400 MHz) δ −57.6.
Compound 3e, 1HNMR (Acetone-d6, 400 MHz) δ 8.12 - 7.22 (m, 8H); 13CNMR (Acetone-d6, 100 MHz) δ 194. 3, 167.8, 166.8, 165.3, 133.8, 133.2, 131.2, 126.4, 116.4; 19FNMR (Acetone-d6, 400 MHz) δ −63.4, −107.1.
Compound 3f, 1HNMR (Acetone-d6, 400 MHz) δ 7.89 - 7.33 (m, 8H); 13CNMR (Acetone-d6, 100 MHz) δ 194.1, 167.5, 165.0, 139.0137.1, 133.6, 132.3, 129.6, 16.5; 19FNMR (Acetone-d6, 400 MHz) δ −107.9.
Compound 3g, 1HNMR (CDCl3, 400 MHz) δ 7.58 - 7.28) (m, 8H), 13CNMR (CDCl3, 100 MHz) δ 194.2, 148.8, 138.7, 138.2, 133.8, 129.0, 128.3, 121.3, 19FNMR (CDCl3, 400 MHz) δ −57.8.
Compound 3h, 1HNMR (CDCl3, 400 MHz) δ 7.79 - 7.22 (m, 8H), 13CNMR (CDCl3, 100 MHz) δ 195.2, 152.1, 137.1, 135.8, 131.9, 129.9, 128.4, 120.2, 19FNMR (CDCl3, 400 MHz) δ −57.6.
Compound 3i, 1HNMR (CDCl3, 400 MHz) δ 8.19 - 7.30 (m, 8H), 2.45 9s, 3H); 13CNMR (CDCl3, 100 MHz) 195.0, 170.8, 153.2, 132.2, 131.8, 130.2, 129.1, 120.2, 21.6; 19FNMR (CDCl3, 400 MHz) δ −57.6.
Compound 3j, 1HNMR (Acetone-d6, 400 MHz) δ 7.36 - 6.91 (m, 8H), 1.96 (s, 3H); 13CNMR (Acetone-d6, 100 MHz) δ 184.5, 134.2, 124.6, 122.8, 122.7, 120.2, 119.8, 106.1, 11.5.
Compound 3k, 1HNMR (CDCl3, 400 MHz) δ 7.83 - 6.97 (m, 8H), 3.90 (s, 3H); 19FNMR (CDCl3, 400 MHz) δ −57.6 [product 3k from potassium (4-methoxy) phenyltrfluoborate and 4-trifluoromethoxy benzoyl chloride was not added in Figure 1 due to limited space].
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Al-Masum, M., Islam, T. and Clopton, G. (2019) Efficient Cross-Coupling Reaction of Aryltrifluoroborates and Aroyl Chlorides for the Synthesis of Fluorine Substituted Aromatic Ketones. International Journal of Organic Chemistry, 9, 67-72. https://doi.org/10.4236/ijoc.2019.91006
References
- 1. Woydziak, Z.R., Fu, L. and Peterson, B.R. (2012) Synthesis of Fluorinated Benzophenones, Xanthones, Acridones, and Thioxanthones by Iterative Nucleophilic Aromatic Substitution. The Journal of Organic Chemistry, 77, 473-481. https://doi.org/10.1021/jo202062f
- 2. Belluti, F., Simone, A.D., Tarozzi, T., Bartolini, M., Djemil, A., Bisi, A.S., Gobbi, S., Montanari, S., Cavalli, A., Andrisano, V., Bottegoni, G. and Rampa, A. (2014) Fluorinated Benzophenone Derivatives: Balanced Multipotent Agents for Alzheimer’s Disease. European Journal of Medicinal Chemistry, 78, 157-166. https://doi.org/10.1016/j.ejmech.2014.03.042
- 3. Stille, J.K. (1978) A General, Selective, and Facile Method for Ketone Synthesis from Acid Chlorides and Organotin Compounds Catalyzed by Palladium. Journal of the American Chemical Society, 100, 3636-3638. https://doi.org/10.1021/ja00479a077
- 4. Labadie, J.W. and Stille, J.K. (1983) Mechanism of the Palladium-Catalyzed Couplings of Acid Chlorides with Organotin Reagents. Journal of the American Chemical Society, 105, 6129-6137. https://doi.org/10.1021/ja00357a026
- 5. Haddach, M. and McCarthy, J.R. (1999) A New Method for the Synthesis of Ketones: The Palladium-Catalyzed Cross-Coupling of Acid Chlorides with Arylboronic Acids. Tetrahedron Letters, 40, 3109-3112. https://doi.org/10.1016/S0040-4039(99)00476-1
- 6. Cho, C.S. (2008) A Recyclable Palladium Catalysis in Cross-Coupling Reactions between Aroylchlorides and Sodium Tetraphenylborate. Catalysis Communications, 9, 2261-2263. https://doi.org/10.1016/j.catcom.2008.05.013
- 7. Al-Masum, M. and Liu, K. (2011) A New Organic Transformation by Introduction of Crotyl/Allyltrifluoroborates in Cross-Coupling Reaction with Aroyl Chlorides. Tetrahedron Letters, 52, 5090-5093. https://doi.org/10.1016/j.tetlet.2011.07.107
- 8. Al-Masum, M. and Wai, E.Ng. (2011) Palladium-Catalyzed Direct Cross-Coupling of Potassium Styryltri-fluuoroborates and Benzoyl Chlorides—A One Step Method for Chalcone Synthesis. Tetrahedron Letters, 52, 1008-1010. https://doi.org/10.1016/j.tetlet.2010.12.085


