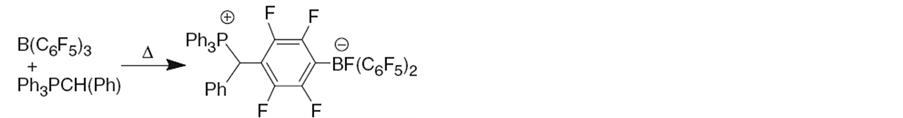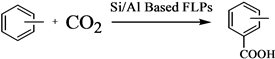Journal of Materials Science and Chemical Engineering
Vol.03 No.01(2015), Article ID:55576,6 pages
10.4236/msce.2015.31015
Carboxylation of Aromatics by CO2 under “Si/Al Based Frustrated Lewis Pairs” Catalytic System
Miaofei Gu, Zhenmin Cheng*
State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
Email: *zmcheng@ecust.edu.cn


Received January 2015

ABSTRACT
Carboxylation of aromatics by CO2 to generate corresponding carboxylic acids is recently providing a novel approach to utilize the green gas CO2, in which the activation of CO2 is the key procedure. Among the many catalytic systems employed in the carboxylation, the concept of “Frustrated Lewis Pairs” (FLPs) was scarcely mentioned, which perform excellently in activating small molecules like CO2. The FLPs are combinations of Lewis acids and Lewis bases which failed to form adducts due to their bulky steric congestion. In this paper, we first attempted various Si/Al Based FLPs to catalyze the carboxylation of aromatics through the activation of CO2, and a good yield of 62% - 97% was obtained. The reaction mechanism was proposed, involving the activation of CO2 mainly contributed by AlCl
Keywords:
Carboxylation, Frustrated Lewis Pairs, Carbon Dioxide, Aromatic, Catalytic System

1. Introduction
Carboxylation of aromatics by CO2 is a competitive alternative to generate corresponding carboxylic acids, in view of its narrow spectra of by-products, effective utilization of greenhouse gas CO2, as well as the mild reacting conditions [1]. Olah et al. [2] first discovered that mixing of benzene with CO2 at 5.7 MPa and 70˚C in the presence of Al/AlCl3 led to benzoic acid as the sole product with 88% in yield, and the pathway of the reaction was studied using DFT calculation, only to find that the carboxylation was proceeded through the formation of “CO2-(AlCl3)n” complexes via activation of CO2, which was then reacting with the aromatics in a typical electrophilic substitution. Various Lewis acids were proved to be catalytic for the carboxylation, among which AlCl3 performs the best (Equation (1)).
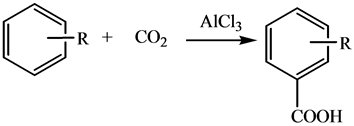 (1)
(1)
More recently, Munshi et al. [3] [4] found that the activation of CO2 by AlCl3 before mixing with toluene could promote the carboxylation remarkably, while Nemoto et al. [5] [6] found the Lewis acid-mediated carboxylation of aromatic compounds with CO2 could be greatly promoted by the addition of a large excess of silyl chlorides, and a good to excellent yield of arylcarboxylic acids was obtained. However, we found that the utilization of FLPs, a novel catalytic system, could significantly accelerate the reaction, and also improve the isolated yield of carboxylic acids.
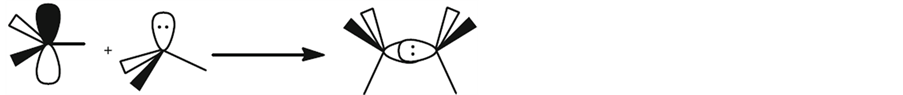
Frustrated Lewis Pairs (FLPs) is now widely used for activation of CO2. The so-called “frustrated Lewis pairs” is a kind of combination consisted of a Lewis acid and a Lewis base which failed to form an adduct due to their bulky steric congestion, or because of the weak attractions between Lewis acid and base, illustrated as Figure 1 and Figure 2.
In 2006, Stephan et al. first observed the activation of H2 using bifunctional phosphinoborane species R2P(C
Figure 1. Classical formation of a Lewis acid-base adduct: Lewis acid + Lewis base → Lewis acid-base adduct [7].
Figure 2. Non-conventional combinations of Lewis acids and bases: FLPs [7].
To the best of our knowledge, there is no research about the CO2 activation in carboxylation of aromatics using FLPs consisted of AlCl3. In this paper, Si/Al Based FLPs were attempted to catalyze the carboxylation of aromatics, such as toluene, ethylbenzene, m-xylene, etc., and a feasible mechanism of carboxylation under Si/Al Based FLPs catalytic system was proposed, involving the activation of CO2 mainly contributed by AlCl
2. Experimental
2.1. Materials and Instruments.
Toluene (99.5%), ethylbenzene (99.5%), m-xylene (99%), mesitylene (97%), benzene (99.5%), CO2 (99.99%), and anhydrous AlCl3 were used for carboxylation, which were commercially purchased and used as received. The anhydrous AlCl3 was handled under inert atmosphere to avoid decomposing by water. Organosilanes R4Si such as Me3SiCl, Me2PhSiCl, MePh2SiCl, Ph3SiCl were also used in carboxylation. HCl (36% - 38%), anhydrous diethyl ether (99.5%) and NaOH (96%) were used for separation of product carboxylic acids.
The instruments used in this work were Nuclear Magnetic Resonance (NMR, Bruker 500), and Infrared Imaging Spectrometer (Nicolet 6700). The carboxylation was carried out in a 100 ml
2.2. Carboxylation of Aromatics to Generate Corresponding Arylcarboxylic Acids
All materials including Al2Cl6 (2.25 mmol, 0.6 g), toluene (20 ml), and Me3SiCl (0.25 g, 2.25 mmol) were added to the autoclave. After pressurization of CO2, the temperature was raised to 80˚C, where the carboxylation was conducted for 12 h until the reaction reached equilibrium. Afterwards, the reaction was stopped by cooling down to room temperature. Water was added to the reacted mixture and the products were extracted with diethyl ether and 10% NaOH. The extracted aqueous phase was adjusted to pH = 1 by adding hydrochloric acid. Precipitated white crystals were obtained after the ice bath cooling.
The toluic acid obtained was qualitatively identified by IR. The ratio of the acid isomers was evaluated by relative integration ratio of1H NMR signals, δH (500MHz, CDCl3, Me4Si) 2.56 (H, s, o-Me), 2.39 (H, s, m-Me), 2.31 (H, s, p-Me). The ratio of ortho, meta, and para was found to be 9:4:87.
Other experiments with different Si/Al Based FLPs were conducted in a similar manner as detailed above, and the contrast experiment employing only AlCl3as catalyst was also conducted.
3. Results and Discussion
3.1. Carboxylation of Toluene by CO2 under Si/Al Based FLPs
Several combinations of Si/Al Based FLPs were selected to carry out the carboxylation of toluene by CO2, to generate corresponding p-toluic acid (p-TA). The results were listed in Table 1. Since the aluminimium chloride tends to dimerize in most organic solvents like toluene, benzene, xylene, etc., here we calculate the isolated yield based on dimer Al2Cl6.
Table 1. Carboxylation of toluene through CO2 activation under Si/Al Based FLPs.
a: Molar ratio R3SiCl/AlCl3 = 1:1. b: Isolated yield is calculated based on Al2Cl6 : p-TA = 1:1. Products obtained by extraction with diethyl ether and NaOH, followed by precipitation in ice bath after adjusted to pH = 1.
According to Table 1, all the Si/Al Pairs were capable to catalyze the carboxylation of toluene by CO2, giving satisfying yields of 62% - 95%. When Ph3SiCl was added accompanied with AlCl3, the p-TA yield reached 95% (entry 4), which exceeded a lot compared to that of only AlCl3 used (78%, entry 5). However, the yield dropped to 62% when employed Me3SiCl/AlCl3 pairs instead (entry 1), suggesting that the catalytic effect is influenced by the steric hindrance of organosilane molecules. This supposition was further demonstrated by the phenomenon of introducing Me2PhSiCl and MePh2SiCl that the p-TA yield is ranked as Ph3SiCl > MePh2SiCl > Me2PhSiCl > Me3SiCl, just corresponds to their size of substituent groups, i.e., the steric hindrance.
As advocate above, the key procedure in carboxylation of aromatics is the activation of CO2, which means the catalytic performance is depended on the activation effect. According to the formation mechanism of FLPs, it is combined by the weak electronic attractions owing to the bulky structure, thus the organosilane which possessed larger substituent group is favorable to form the FLPs with AlCl3, leading to a high efficiency in activation of CO2. The activation process is shown in Figure 3.
3.2. Activation of CO2 in Carboxylation of Aromatics
Other alkylbenzenes were carboxylated with the acid of different Si/Al Based FLPs, and we proposed a feasible mechanism of carboxylation under Si/Al Based FLPs catalytic systems, involving the activation of CO2 mainly contributed by AlCl
The reaction is believed to be an electrophilic aromatic substitution (SEAR) by an attack to the aromatics nucleus of the activated “CO2-AlCl3-R4Si”. It is known that a trialkysilyl group on the aromatics nucleus promotes the SEAR reaction, as the silyl group stabilizes the transition state, leading to the (p-σ)π conjugation between the Si-C bond and the formation of a benzenonium intermediate. Suspension of AlBr3 and Ph3SiCl in cyclohexane to CO2reveals a C=O stretching vibration, assigned to a species consisting of CO2, AlBr3, and Ph3SiCl [6]. This suggests that organosilane could activate CO
Figure 3. Activation of CO2 using different Si/Al Based FLPs.
Figure 4. Feasible mechanism of carboxylation of aromatics through activation of CO2.
Table 2. Activation of CO
a: Molar ratio R3SiCl/AlCl3 = 1:1. b: Isolated yield is calculated based on Al2Cl6 : p-TA = 1:1. Products obtained by extraction with diethyl ether and NaOH, followed by precipitation in ice bath after adjusted to pH = 1.
4. Conclusion
Various combinations of Si/Al Based FLPs were employed to catalyze the carboxylation of aromatics, such as toluene, ethylebenzene, m-xylene, etc., and the results indicated that all of the catalytic systems could activate CO2 with a satisfying carboxylic acid yield. A feasible mechanism was proposed, involving the activation of CO2 mainly contributed by AlCl
Acknowledgements
Financial support from the Fundamental Research Funds for the Central Universities is gratefully acknowledged.
Cite this paper
Miaofei Gu,Zhenmin Cheng, (2015) Carboxylation of Aromatics by CO2 under “Si/Al Based Frustrated Lewis Pairs” Catalytic System. Journal of Materials Science and Chemical Engineering,03,103-108. doi: 10.4236/msce.2015.31015
References
- 1. Arakawa, H., Aresta, M., Armor, J.N., Barteau, M.A., Beckman, E.J., Bell, A.T., et al. (2001) Catalysis Research of Relevance to Carbon Management: Progress, Challenges, and Opportunities. Chemical Reviews, 101, 953-996. http://dx.doi.org/10.1021/cr000018s
- 2. Olah, G.A., T?r?k, B., Joschek, J.P., Bucsi, I., Esteves, P.M., Rasul, G. and Surya, P.G. (2002) Efficient Chemoselective Carboxylation of Aromatics to Arylcarboxylic Acids with a Superelec-trophilically Activated Carbon Dioxide- Al2Cl6/Al System. Journal of the American Chemical Society, 124, 11379-11391. http://dx.doi.org/10.1021/ja020787o
- 3. Munshi, P. and Beckman, E.J. (2009) Effect of Incubation of CO2 and Lewis Acid on the Generation of Toluic Acid from Toluene and CO2. Industrial & Engineering Chemistry Research, 48, 1059-1062. http://dx.doi.org/10.1021/ie801524e
- 4. Sarve, A.N., Ganeshpure, P.A. and Munshi, P. (2012) Carboxylation of To-luene by CO2 Generating p-Toluic Acid: A Kinetic Look. Industrial & Engineering Chemistry Research, 51, 5174-5180. http://dx.doi.org/10.1021/ie300014z
- 5. Nemoto, K., Yoshida, H., Suzuki, Y., Morohashi, N. and Hattori, T. (2006) Beneficial Effect of TMSCl in the Lewis Acid-Mediated Carboxylation of Aromatic Compounds with Carbon Dioxide. Chemistry Letters, 35, 820-821. http://dx.doi.org/10.1246/cl.2006.820
- 6. Nemoto, K., Yoshida, H., Egusa, N., Morohashi, N. and Hattori, T. (2010) Direct Carboxylation of Arenes and Halobenzenes with CO2 by the Combined Use of AlBr3 and R3SiCl. The Journal of Organic Chemistry, 75, 7855-7862. http://dx.doi.org/10.1021/jo101808z
- 7. Stephan, D.W. (2013) Discovery of Frustrated Lewis Pairs: Intermolecular FLPs for Activation of Small Molecules. Topics in Current Chemistry, 332, 1-44. http://dx.doi.org/10.1007/128_2012_381
- 8. Welch, G.C., San Juan, R.R., Masuda, J.D. and Stephan, D.W. (2006) Reversible, Metal-Free Hydrogen Activation. Science, 314, 1124-1126.
- 9. Stephan, D.W. (2008) Frustrated Lewis pairs: a Concept for New Reactivity and Catalysis. Org. Biomol. Chem., No. 6, 1535-1539.
- 10. Stephan, D.W. (2010) Activation of Dihydrogen by Non-Metal Systems. Chem. Comm., No. 46, 8526-8533.
- 11. Erker, G. (2011) Organo-metallic Frustrated Lewis Pair Chemistry. Dalton Trans., No. 40, 7475-7483.
- 12. Ashley, A.E., Thompson, A.L. and O’Hare, D. (2009) Non-Metal-Mediated Homogeneous Hydrogenation of CO2 to CH3OH. Angew Chem. Int. Ed, 48, 9839-9843. http://dx.doi.org/10.1002/anie.200905466
- 13. Berkefeld, A., Piers, W.E. and Parvez, M. (2010) Tandem Frustrated Lewis Pair/tris(pentafluorophenyl)borane-catalyzed Deoxygenative Hydrosilylation of Carbon Dioxide. Journal of the American Chemical Society, 132, 10660-10661. http://dx.doi.org/10.1021/ja105320c
- 14. Appelt, C., Westenberg, H., Bertini, F., Ehlers, A.W., Slootweg, J.C. and Lammertsma, K. (2011) Geminal Phosphorus/Aluminum-Based Frustrated Lewis Pairs: C-H Versus C-C Activation and CO2 Fixation. Angewandte Chemie, 50, 4011-4014. http://dx.doi.org/10.1002/ange.201006901
- 15. Peuser, I., Neu, R.C., Zhao, X., Ulrich, M., Schirmer, B., Tannert, J.A., et al. (2011) CO2 and Formate Complexes of Phosphine/Borane Frustrated Lewis Pairs. Chemistry—A European Journal, 17, 9640-9650. http://dx.doi.org/10.1002/chem.201100286
- 16. Andrew, E., Ashley, A.E. and O’Hare, D. (2013) FLP-Mediated Activations and Reductions of CO2 and CO. Top Curr. Chem., 334, 191-218.
- 17. Momming, C.M., Otten, E., Kehr, G., Frohlich, R., Grimme, S., Stephan, D.W. and Erker, G. (2009) Reversible Metal- Free Carbon Dioxide Binding by Frustrated Lewis Pairs. Angewandte Chemie International Edition, 48, 6643-6646. http://dx.doi.org/10.1002/anie.200901636
NOTES

*Corresponding author.