Journal of Biosciences and Medicines
Vol.03 No.07(2015), Article ID:57825,22 pages
10.4236/jbm.2015.37005
Efficacy of Some Antibiotics in Curing Resistant Escherichia coli Infection
Tarek El-Banna1, Ahmed Abd El-Aziz1, Nageh EL-Mahdy2, Yasmine Samy3*
1Microbiology Department, Faculty of Pharmacy, Tanta University, Tanta, Egypt
2Pharmacology Department, Faculty of Pharmacy, Tanta University, Tanta, Egypt
3Pharmaceutical Sciences, Faculty of Pharmacy, Tanta University, Tanta, Egypt
Email: t_elbanna@yahoo.com, ahaaziz@gmail.com, nagehmahdy@hotmail.com, yasmine_samy8080@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 18 May 2015; accepted 4 July 2015; published 9 July 2015
ABSTRACT
There is growing interest in re-evaluation of older antibiotics with the wide spread of pathogen resistance, especially gram negative bacteria, which impair treatment of some infections. In contrast various studies have reported that some antibiotics have efficacy in clearing resistant bacterial infections. On account of that it was interesting to evaluate the efficacy of erythromycin, chloramphenicol and/or tenoxicam in curing and/or relieving wound infection of highly resistant Escherichia coli and investigate the possible mechanisms beyond their antibacterial activity. This was achieved through evaluating highly resistant E. coli strains in vitro using agar dilution and in vivo rat models of E. coli infected wound and acute inflammation by carrageenin, where possible mechanisms were evaluated through measuring immunological mediators and histopathological examination. This study revealed that in vivo, erythromycin alone or in combination with tenoxicam significantly improved the healing of infected skin wounds with E. coli irresspective of resistancy in vitro. In addition to the improvement of immunological mediators involved in inflammatory reaction, oxidative stress and in cytokines expression as response to the bacterial infection in vivo. On the other hand chloramphenicol neither alone nor in combination with tenoxicam, achieved any significant effect. Tenoxicam didn’t show antimicrobial activity alone nor in combination with tested antibiotics in vitro, but it has shown synergestic activity in combination with tested antibiotics in vivo. Thus we concluded that immunomodulatory activity of erythromycin through anti-inflammatory and antioxidant effects was the possible mechanisms by which this antibiotic had healed infection with resistant E. coli in vivo, despite its resistancy to this antibiotic in vitro.
Keywords:
Efficacy, Antibiotics, Resistant, E. coli, Infection

1. Introduction
Resistance to antibiotics has increased dramatically over the past few years and has now reached a level that places future patients in real danger [1] . Such increasing antimicrobial drug resistance of bacterial pathogens, together with the relative shortage of new antimicrobial agents, calls for a new look at the therapeutic options [2] [3] .
In contrast various studies have reported that some antibiotics have curing activity of infections caused by resistant bacteria although absence of suscebetibility to those antibiotics, due to other mechanisms beyond their antimicrobial activity, such as reported by Tsai et al. (2009) and Imperi et al. (2014) [4] [5] .
On accont of that several investigators have suggessted re-evaluation of old antibiotics through further understanding and investigation of their mechanism(s) of action(s) [6] - [8] . It is worth reminding the clinician that it is necessary to be far more selective, when an antibiotic is prescribed, it should be the one with the narrower spectrum of activity [9] .
Accordingly, evaluation of the efficacy of antibacterial agents requires criteria other than those defined in vitro, such as minimum inhibitory concentrations (MICs) or minimum bactericidal concentration (MBCs). In the last years, we have seen a renewal of interest in another approach to the successful resolution of infection: the achievement of synergestic interaction between therapeutic agents and the host defense system [10] [11] .
With a growing number of supportive experimental and clinical studies, the relevance of the immunomodulatory actions of some antibiotics for their therapeutic efficacy in various diseases is now generally admitted [11] . This is coincided with the fact that the immune system response to a microorganism may be beneficial in infection, and has the potential to be more devastating than direct damage caused by a microbe, but facilitated resolution of the response is needed to limit tissue damage [12] [13] . So that the potential benefits of down regulating immunomodulators entered the limelight, with the understanding that immune hyper-activation for example in sepsis, can have disastrous consequences [11] .
Despite advances in operative techniques and a better understanding of the pathogenesis of wound infection and wound healing, post operative wound infection continues to be a major source of morbidity and mortality for patients undergoing operative procedures [14] . Concurrently the major concern for clinicians in the treatment of infectious disease is the bacterial biofilm because of the resistance to a wide range of antibiotics [15] [16] . Therefore, a better understanding of bacterial biofilms is needed to develop novel therapeutics for the prevention and treatment of wound infections [17] [18] . Also adjustment of the given dose of antibiotics is critical in removal of biofilm [19] .
An animal model of wound colonization generated in a manner similar to the wound infection model would be useful to study the pathophysiology of wound infections and monitor the effect of therapeutic agents in vivo [20] .
Escherichia coli multiple-antibiotic-resistance (MAR) mutants are resistant to a wide variety of antibiotics [21] . E. coli is one of the most causative microorganisms in post operative wound infections [14] [22] .
This study aimed to evaluate efficacy of erythromycin and chloramphenicol alone and in combination with tenoxicam and investigate the possible mechanisms of these antibiotics beyond their antimicrobial activity in curing resistant E. coli infected wound using in vivo rat models.
2. Materials & Methods
2.1. Materials
Antimicrobial agents: Erythromycin (Abott, USA) and Chloramphenicol (Sigma, USA) were used.
Bacterial strains: A total of thirty clinical isolates of E. coli were obtained from Microbiology Department, Faculty of Pharmacy, Tanta University, Egypt. Purity and identity of the clinical isolates were achieved by further identification, according to standard methods directed by Collee et al. (1996) and Koneman et al. (2006) [23] [24] .
Reference strain: Escherichia coli ATCC 25922 standard strain was obtained from NAMRU3, Cairo, Egypt.
Animals: A total of 136 adult male albino rats obtained from the animal house of the National Research Center (NRC), Egypt, weighing from 150 - 200 g were used in the present study. They were fed standard pellet chow (El-Nasr Chemical Company, Cairo, Egypt) and allowed free access to water. Animals were housed in the same conditions for one week prior to the experiment for acclimatization and they were fasted for 12 hr before experimental procedures but allowed free access to water.
Kits: Enzyme Linked Immunosorbant Assay (ELISA) was utilized to examine the concentration of cytokines [25] . Tumor necrosis factor-alpha (TNF-α) ELISA kit (AssayMax, Ireland) and Interleukin-1 Beta (IL-1β) (ELISA) kit (Cusabio Biotech, China) were used.
Drugs used in research: Tenoxicam used as standard non steroidal anti-inflammatory drug (NSAID) and vitamin C used as standard antioxidant drug.
Chemicals: Bis-(3-carboxy-4-nitrophenyl) disulphide (Ellman’s Reagent) (Sigma, USA); Carrageenan sodium (Sigma, USA); Congo red (Sigma, USA); Crystal violet (Beecham, England); Dimethyl sulphoxide (DMSO) (BDH, England); Ethanol (Prolabo, France); Ferric chloride (BDH, England); Glacial acetic acid (Prolabo, France); Hydrochloric acid (HCL) (Prolabo, France); Hydrogen peroxide (Adwic, ARE); Hematoxylin and Eosin (H & E) (Sigma, USA); Iodine (BDH, England); Methyl red (Adwic, ARE); Monohydrogen disodium phosphate (BDH, England); n-Butanol (Prolabo, France); N-(1-naphthyl)ethylenediamine Dihydrochloride (Sigma, U.S.A); Phenol (Sigma, USA); Potassium iodide (Prolabo, France); Reduced Glutathione (GSH) (BDH, England); Safranine (Beecham, England); Sodium chloride (Adwic, ARE); Sodium hydroxide (NaOH) (Sigma, USA); Sodium nitrite (El-Nasr, Egypt); Sulfanilamide (Winlab, England); Sulfosalicylic acid (Fluka Biochemica, Switzerland); 1,1,3,3-Tetramethoxy propane (Sigma, USA); Thiobarbituric acid (Sigma, USA); Thiopental sodium (Eipico, ARE); Trichloroacetic acid (Winlab, England); Vanadium (III) chloride (Sigma, USA).
2.2. Methods
2.2.1. Screening of Highly Resistant Strains of Bacterial Clinical Isolates
This was performed through determination of MIC of the tested antibiotics against all isolates by agar dilution method according to the procedure described by CLSI (2010) and Cursino et al. (2005) [26] [27] . MIC breakpoints for E. coli [26] : Erythromycin (R ≥ 8) and Chloramphenicol (R > 32).
2.2.2. Investigation the Effects of Tenoxicam/Antibiotics Combinations against the Selected MAR E. coli Strain
This was achieved by determination of MIC of selected antimicrobial agents in the presence of serum plasma concentration of tenoxicam (30 μM) [28] , its ½ fold, its 10 fold and its 100 fold against corresponding concentrations of MIC, ½ MIC, ¼ MIC, 1/8 MIC of the tested antibiotics, on MAR E. coli strain. This was performed using agar dilution method according to the procedure described by CLSI (2010) and Cursino et al. (2005) [26] [27] . The fractional inhibitory concentration (FIC) was used to interpret the results of agar dilution method and calculated according to Makay et al. (2000) and Odds (2003) [29] [30] as follows:
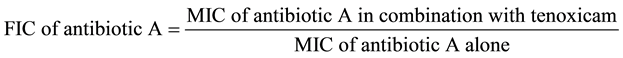
The interaction was recorded as synergism (S) when FIC < 0.5, indifference (I) when FIC > 0.5 to 4, and antagonism (A) when FIC > 4.
2.2.3. Studying the Efficacy of Tested Antibiotics, Tenoxicam and Their Combinations on Healing of Resistant Bacterial Infected Wound in Animal Model
This was achieved as described by Lau et al. (2009) [31] .
A total of 56 adult male rats (about 150 gm/each) were used in this model, they were divided into 1 naiive; 1 infected control and 5 treatment groups, each group was composed of eight rats. One rat of each of the 7 groups was sacrificed on day 3 of the experiment for the histopathological studies to confirm the formation of the bacterial biofilm within the wound bed.
-Formation of wound in rat hind paw: On the day of wound induction (defined as day 0) each rat was anesthetized with an intraperitoneal injection (I.P) of 50 mg/kg thiopental sodium [18] . A rectangular pattern was marked on the dorsal surface of the foot using a flexible transparent plastic template, and then a layer of skin in full thickness with standard area of 2 mm × 5 mm was removed, Figure 1. The initial wound size was measured on day 1 [31] .
-Bacterial inoculum and induction of wound infection: The bacterial inoculation was carried out one day after the wounding development to reduce mortality due to bacteremia in the bleeding phase [20] .
Overnight culture of highly resistant E.coli strain was used to inoculate the wound in rat hind paw. After bacterial inoculation, wounds were left for 48 hrs to allow biofilm formation [32] .
Figure 1. Steps of making wound on the dorsal surface of the rat hind paw.
-Antimicrobial therapy and dosage regimen: All tested antimicrobial agents and tenoxicam, each alone and in combinations were administered I.P except erythromycin was administered orally by gavage.
The 5 treated animal groups in addition to the two: naiive and infected control groups were handled as follows:
I-Naïve group: It consists of normal rats not receiving any treatment.
II-Infected control group: Included animals infected with selected MAR E .coli strain and left 9 days post infection without treatment.
III-Tenoxicam treated group: This group of rats were treated with tenoxicam (standard NSAID), in a dose of (20 mg/kg) [33] .
IV-Erythromycin treated group: animals treated with erythromycin (800 mg/kg) [34] .
V-Erythromycin/tenoxicam combination group: animals treated with erythromycin (400 mg/kg)/tenoxicam (10 mg/kg) combination.
VI-Chloramphenicol group: animals were treated with chloramphenicol (300 mg/kg) [35] .
VII-Chloramphenicol/tenoxicam combination group: animals were treated with chloramphenicol (150 mg/ kg)/tenoxicam (10 mg/kg) combination.
The antibiotics were given to the treated groups in equally divided doses at 6-hour intervals and the tenoxicam was given once daily for 9 days.
Evaluation of wound healing: Observations of wound surface provided information on the gross extent of wound healing and improved wound healing by facilitating tissue regeneration [31] . In this study we selected the one-point sampling method because of the relatively small size of wounds that can be induced in rats and to better avoid contamination with peri-wound flora [18] .
Digital photographs for the wound were taken on days 1, 3, 6 and 11. Wound area measurement was made by using the digital photographs of the wounds. Perior to picture taking, the rat hind paw was gently pinched manually, so that the wound site was positioned at the intersection of the vertical and horizontal grid lines on the graphic paper on to which vertical and horizontal rulers had been fixed [36] , which allowed us to enlarge the photograph in order to increase the accuracy of wound area measurement and the photographs were processed by using Microsoft Word where the wound area was traced and measured on the scale present in the same photograph [18] .
2.2.4. Assessment of Possible Mechanisms of Tested Antibiotics beyond Their Antimicrobial Activity Using Acute Inflammatory Animal Model
This was done through induction of acute inflammation in rats to evaluate anti-inflammatory and anti-oxidant activity of tested antimicrobials beyond their antibacterial activity, using recommended doses of tested antibiotics in the model of carrageenan induced hind paw oedema in rats.
Carrageenan-induced paw inflammation rat model was used for inflammation study and evaluation of anti- inflammatory activity [31] [37] . Carrageenan is sulfated polysaccharide derived from marine algae [38] . It is commonly used to produce short-lasting acute inflammation in animal models [39] . This model was performed according to method adopted by Winter et al. (1962) and Fath et al. (1984) [40] [41] .
Animal groups of carrageenin acute inflammatory model: A total of 80 rats (about 150 gm/each), were used in this model. Antibiotics were administered I.P prior to carrageenin injection, except erythromycin was administered O.P. Rats were allocated into the following groups:
i-Naïve group: Including normal animals without any treatment but were injected with (0.1 ml) of saline only into the subplantar region of the right hind paw of rats and serve as control group.
ii-Carrageenan control group: Rats were slightly anesthetized with ether and 0.1 ml of 1.5% (carrageenan sodium solution in 0.9% saline) was injected S.C into the subplantar region of the right hind paw of rats. Thus oedema will be produced acutely into the right hind paw of the rat, and the left hind paw (used as self control), was injected with similar volume (0.1 ml) of saline only. Those rats did not receive any treatment.
iii-Tenoxicam treated group: Tenoxicam (20 mg/kg,) I.P [33] , was taken 30 minutes before carrageenan injection [42] .
iv-Vitamin C treated group: Vitamin C has antioxidant and anti-infalmmatory activities. It was taken I.P in a dose of 500 mg/kg [43] - [45] . It was used for comparison.
v-Erythromycin treated groups: It is divided into three subgroups according to erythromycin concentrations (200, 400, 800 mg/kg respectively) O.P.
vi-Chloramphenicol treated groups: It is divided into three subgroups according to chloramphenicol concentrations (75, 150, 300 mg/kg respectively) I.P.
2.2.5. Assessment of Immunological Mediators
In both animal models (E. coli infected wound and acute inflammation by carrageenin), the immunological mediators that resulted due to response to the bacterial infection and acute inflammation were assessed, such as TNF-α, IL-1β in rat serum using ELISA kits), NO, GSH and MDA (in rat hind paw tissue according to standard methods directed by Miranda et al. (2001); Ellman (1959) and Yoshioka et al. (1979) respectively [46] - [48] .
2.2.6. Histopathological Examination
Histopathological Examination of rat hind paw sections through sectioning and staining with Hematoxylin and Eosin (H & E) was achieved using standard methods adopted by Bancroft & Stevens (1975) [49] .
In bacterial infected wound animal model, biopsy specimens were obtained 48 hours after inoculation and colonization to ensure the biofilm formation, and at the end of the treatment of each group in addition to naiive and infected control group for examination with light microscope. Specimens were placed in buffered 10% formaldehyde for fixation and stained with H & E and Gram crystal violet [18] [32] [50] .
In Carrageenin hind paw oedema animal model, all treatment groups in addition to naiive and carrageenin control group in carrageenin model, the rat hind paw was removed, washed with saline, immediately fixed in 10% buffered formalin solution (pH 7.4) for 24 hrs and then routinely processed in ascending grades of alcohol, then xylene. The tissues were then embedded in paraffin wax, serially-sectioned to (3 - 5 mm) thickness, and stained with H & E.
Finally, each stained tissue section was examined using a light microscope (Olympus BX 51, Olympus America, Melville, NY) and photographed with a digital camera (Olympus DP11) connected to the microscope.
2.2.7. Statistical Analysis
Minitab computer software (version 16) was used to carry out the statistical analysis. Results were expressed as the mean ± SE of mean and analysed using Student t-test. Comparisons between different groups were carried out by one way analysis of variance (ANOVA). The level of significance was considered as non-significant difference if p ≥ 0.05, on the other hand, if p < 0.05, this indicates a significant difference.
3. Results
3.1. Screening of Resistant Strains
Tested E. coli isolates expressed high level of resistance to the studied antibiotics, their MICs values were (50%) 64 μg/ml; (25%) 256 μg/ml and (25%) 512 μg/ml for erythromycin but (90%) 1024 μg/ml and 10% > 1024 (μg/ml) for chloramphenicol.
The assessment of antimicrobial activity of tenoxicam revealed that tenoxicam didn’t have antimicrobial activity against any tested isolates nor the reference strain.
Thus from these in vitro experiments, it was noted worthy that combinations of tested antibiotics with tenoxicam, were indifferent (FIC = 1) from using those antibiotics agents alone against MAR E. coli isolates.
3.2. The Efficacy of Tested Antibiotics, Tenoxicam and Their Combinations on Healing of Resistant Bacterial Infected Wound in Animal Model
The wound healing effects of erythromycin, chloramphenicol and/or tenoxicam of rat hind paw infected with resistant E. coli strain, are shown in the following Figure 2(i) and Figure 2(ii), of the wound area photos were taken on days 1, 3, 6 and 11.
It was obvious that in erythromycin alone and in combination with tenoxicam or tenoxicam treated groups, there were greater reduction in the ulcer area than was observed in those groups treated with chloramphenicol and untreated infected control group. The average ulcer area in the untreated infected control group, chloramphenicol alone and with tenoxicam combination groups, decreased from 14 mm2 on day 1 to 6 mm2 on day 9. On the other hand the average ulcer area in treatment groups of erythromycin and/or tenoxicam decreased from 12 mm2 on day 1 to 0 mm2 (complete healing) on day 7.
Here it was noticed that only 7 days were required for complete healing of wound infection in erythromycin treated groups both alone or in combination with tenoxicam. While longer time was required in groups of infected control (without treatment), treatment with chloramphenicol alone and in combination with tenoxicam.
Figure 2. (i) Effects of the treatment with erythromycin and/or tenoxicam on wound healing of rat hind paw infected with MAR E. coli; (ii) Effect of the treatment with chloramphenicol alone and with tenoxicam on wound healing of rat hind paw Infected with resistant E. coli and the infected control (untreated) group.
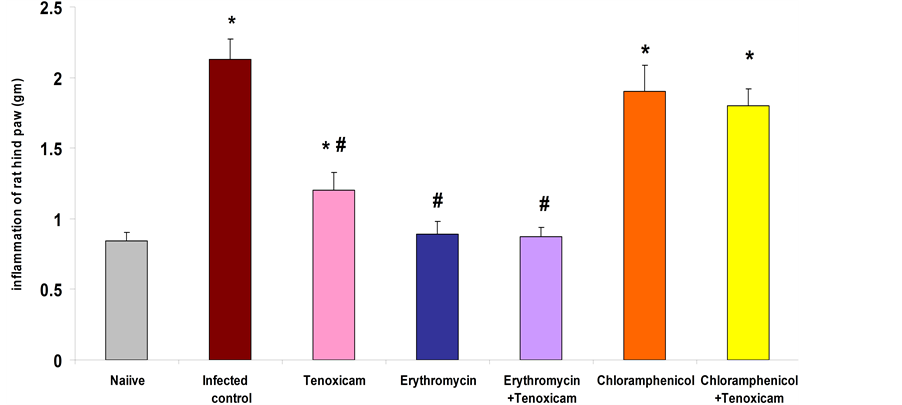
3.3. The Effect of Tested Antibiotics and/or Tenoxicam on the Inflammation of Rat Hind Paw Infected with MAR E. coli Strain
Obtained data revealed that rats in infected control group, showed a significant (p < 0.001) increase (252.1%) in weight of rat hind paw compared to naïve group, while treated rats with erythromycin and erythromycin/te- noxicam combination resulted in a significant (p < 0.001) reduction (58.2% and 58.9% respectively) in hind paw weight as compared to the infected control group. Treatment of rats with tenoxicam resulted in a significant (p < 0.001) reduction (43.7%) in hind paw weight as compared to the infected control but treatment of rats with chloramphenicol and chloramphenicol/tenoxicam combination resulted in an insignificant reduction (10.8% and 15.5% respectively) in hind paw weight as compared to the infected control (Figure 3).
3.4. The effect of Tested Antibiotics and/or Tenoxicam on Cytokines Content in Serum of rats Infected with MAR E. coli Strain
Studied cytokines showed that IL-1β content in rat serum significantly (p < 0.001) increased (1603.4%) in infected control group as compared to naïve group; treatment of rats with erythromycin and erythromycin/tenox- icam combination resulted in a significant (p < 0.001) reduction (95.06% and 95.2% respectively) in IL-1β content in rat serum as compared to the infected control group. while treatment of rats with tenoxicam alone resulted in a significant (p > 0.001) reduction (50.3%) in IL-1β content in rat serum as compared to the infected control group. Treatment of rats with chloramphenicol and chloramphenicol/tenoxicam combination resulted in an insignificant (p > 0.05) reduction (15.6% and 23.8% respectively) in IL-1β content in rat serum as compared to the infected control group, Figure 4(a).
TNF-α content in serum of rats of infected control group showed a significant(p < 0.001) increase (1174.8%) as compared to naïve group. treatment of rats with erythromycin and erythromycin/tenoxicam combination resulted in a significant (p < 0.001) reduction (93.9% and 89.7% respectively) in TNF- α content in rat serum as compared to the infected control. On the other hand treatment of rats with chloramphenicol and chloramphenicol/tenoxicam combination resulted in an insignificant (p > 0.05) reduction (16.03% and 31.7% respectively) in TNF-α content in rat serum as compared to the infected control. Also treatment of rats with tenoxicam resulted in a significant (p < 0.05) reduction (40%) in TNF-α content in rat serum as compared to the infected control, Figure 4(b).
Figure 3. Effect of erythromycin (800 mg/kg); tenoxicam (20 mg/kg); erythromycin (400 mg/kg)/tenox- icam (10 mg/kg) combination; chloramphenicol (300 mg/kg) and chloramphenicol (150 mg/kg)/tenox- icam (10 mg/kg) combination on inflammation of rat hind paw infected with resistant E. coli. *Significant from naïve at p < 0.001 for infected control, erythromycin, erythromycin/tenoxicam, chloramphenicol/ tenoxicam combination groups, at p < 0.01 for chloramphenicol and at p < 0.05 for tenoxicam group. #Significant from infected control at p < 0.001. The results are means ± S.E.M.


Figure 4. (a) Effects of erythromycin (800 mg/kg), tenoxicam (20 mg/kg), erythromycin (400 mg/kg)/tenoxicam (10 mg/kg) combination, chloramphenicol (300 mg/kg) and chloramphenicol (150 mg/kg)/tenoxicam (10 mg/kg) combination on IL-1β content in serum of rats infected with MAR E. coli. *Significant from naïve at p < 0.001 and at p < 0.01 for tenoxicam. #Significant from infected control at p < 0.001. The results are means ± S.E.M. (b) Effects of erythromycin (800 mg/kg), tenoxicam (20 mg/kg), erythromycin (400 mg/kg)/tenoxicam (10 mg/kg) combination, chloramphenicol (300 mg/kg) and Chloramphenicol (150 mg/kg)/tenoxicam (10 mg/kg) combination on TNF-α content in serum of rat infected with MAR E. coli. *Significantly different from naiive at p < 0.01. #Significantly different from infected control at p < 0.01, tenoxicam at p < 0.05. The results are means ± S.E.M.
3.5. The Effect of Tested Antibiotics and/or Tenoxicam on Other Immunological Mediators in Rats Infected with MAR E.coli Strain
NO content of rat hind paw significantly increased in infected control group rats by (412.2%) as compared to naïve group, but erythromycin and erythromycin/tenoxicam combination treated groups have shown a significant (p < 0.001) reduction (75% and 76% respectively) in hind paw NO content as compared to the infected control; while chloramphenicol and chloramphenicol/tenoxicam combination treated groups have shown an insignificant (p > 0.05) reduction (13.6% and 20% respectively) in hind paw NO content as compared to the infected control; also tenoxicam treaed rats resulted in a significant (p < 0.01) reduction (48.8%) in hind paw NO content as compared to the infected control Figure 5(a).
GSH content of rat hind paw in infected control group showed a significant (p < 0.001) decrease (92.8%) as compared to naïve group. Treatment of rats with erythromycin and erythromycin/tenoxicam combination resulted in a significant (p < 0.001, p < 0.01 respectively) increase (388.1% and 402.2% respectively) in hind paw GSH content as compared to the infected control group; while treatment of rats with chloramphenicol resulted in an insignificant (p > 0.05) increase (51.7) but chloramphenicol/tenoxicam combination significantly (p < 0.05) increase (63.8%) in hind paw GSH content as compared to the infected control group on the other hand treatment of rats with tenoxicam resulted in a significant (p < 0.001) increase (394.4%) in hind paw GSH content as compared to the infected control group, Figure 5(b).
MDA content of rat hind paw in infected control group showed a significant (p< 0.001) increase (527.9%) as compared to naïve group. Treatment of rats with erythromycin and erythromycin/tenoxicam combination resulted in a significant (p < 0.001) reduction (79.1% and 80.4% respectively) in hind paw MDA content as compared to the infected control. Treatment of rats with chloramphenicol resulted in an insignificant (p > 0.05) reduction (15.2%) but chloramphenicol/tenoxicam resulted in significant (p < 0.01) reduction (27%) in hind paw MDA content as compared to the infected control. Treatment of rats with tenoxicam resulted in a significant (p< 0.001) reduction (63.2%) in hind paw MDA content as compared to the infected control Figure 5(c).



Figure 5. The results are means ± S.E.M. (a): Effects of erythromycin (800 mg/kg), tenoxicam (20 mg/kg) and erythromycin (400 mg/kg)/ tenoxicam (10 mg/kg) combination, chloramphenicol (300 mg/kg) and chloramphenicol (150 mg/kg)/tenox- icam (10 mg/kg) combination on NO content of rat hind paw infected with resistant E.coli. *Significant from naïve at p < 0.001 for all groups and for tenoxicam significant at p < 0.05. #Significant from infected control at p < 0.001 for all groups, but for tenoxicam at p < 0.01; (b) Effects of erythromycin (800 mg/kg), tenoxicam (20 mg/kg), erythromycin (400 mg/kg)/ tenoxicam (10 mg/kg) combination, chloramphenicol (300 mg/kg) and chloramphenicol (150 mg/kg)/tenoxicam (10 mg/kg) combination on GSH of rat hind paw infected with MAR E. coli. *Significant from naïve at p < 0.001. #Significant from infected control at p < 0.001 but for erythromycin/tenoxicam at p < 0.01 and chloramphenicol/tenoxicam at p < 0.05; (c) Effects of erythromycin (800 mg/kg), tenoxicam (20 mg/kg) and erythromycin (400 mg/kg)/tenoxicam (10 mg/kg) combination, chloramphenicol (300 mg/kg) and chloramphenicol (150 mg/kg)/tenoxicam (10 mg/kg) combination on MDA of rat hind paw infected with MAR E. coli. *Significant from naïve at p < 0.001. #Significant from infected control at p < 0.001 but for chloramphenicol/tenoxicam combination at p < 0.01.
3.6. Acute Inflammatory Model (Carrageenin Induced Hind Paw Oedema in Rats)
This model was performed to illustrate the possible mechanisms beyond the antimicrobial activity of tested antibiotics, by which they healed infected wounds despite resistancy of E. coli strain used in infection, through assessing the anti-inflammatory and anti-oxidant activities of different doses for those antibiotics. Tenoxicam and vitamin C were used as standard anti-inflammatory and antioxidant respectively for comparison. The results of tested immunological parameters in this model were shown in Table 1.
3.7. Histopathological Studies
These studies were performed to confirm effects of tested antibiotics on immunological markers of both in vivo models, which revealed that all animals of naiive group showed normal cutaneous structure regarding epidermis and dermal connective tissue structures, normal skin appendages (sweat glands) Figure 6(a).
Infected animal model: After 48 hours of wound infection with E. coli before treatment (to allow formation of bacterial biofilm on wound of rat hind paw), there were signs of acute inflammation manifested as severe oedema, prominent capillary diltation and prevascular inflammatory cellular infiltration associated with severe dense interstitial acute inflammatory cellular infiltration down in deep dermis Figure 6(b).
Infected group without treatment showed mild relief of acute signs (decrease in oedema and density of acute inflammatory infiltration with early fibrosis) Figure 6(c).
Tenoxicam treated group showed decrease of inflammatory reaction signs Figure 6(d).
Erythromycin and erythromycin/tenoxicam combination treated groups showed healing with dermal fibrosis Figure 6(e).
Chloramphenicol treated group showed mild inflammatory infiltration and mild oedema Figure 6(f) but chloramphenicol/tenoxicam Combination treated group showed healing with dermal fibrosis. Figure 6(g).
Carrageenin acute inflammatory (noninfected) animal model: Carrageenin control group, all studied animals showed severe dermal dense acute inflammatory cellular infiltration (dilated, engorged capillaries with prominent endothelial swelling with oedema) Figure 6(h).
In both of tenoxicam and vitamin C groups (used for comparison as standard (NSAID) and standard antioxidant respectively), all studied animals showed apparently normal sections as naïve group with early inflammatory changes manifested as mild oedema with dilated, congested capillaries and normal covering epidermis Figure 6(i).
In Erythromycin treated groups, all animals showed healing with dermal fibrosis Figure 6(e).
Chloramphenicol treated groups, all animals showed epidermal thickening (akanthosis, parakeratosis, hyperkeratosis), oedema and fibrosis in the dermis with mild to moderate inflammatory infiltration Figure 6(j).
Table 1. Efficacy of tested antibiotics and/or tenoxicam on studied parameters in acute inflammatory carrageenin model in relation to carrageenin control.
Data are presented as mean (±S.E.M; n = 8/group). *Significant from naïve at p < 0.001. **Significant from naïve at p < 0.01. #Significant from carrageenin control at p < 0.001. ##Significant from carrageenin control at p < 0.01.





Figure 6. (a) showing normal epidermal covering layers and the normal dermis showing no inflammatory reactions (H & E ×250); (b) showing dense acute inflammatory cellular infiltration down to the deep dermis with ulceration and pyogenic membrane formation (H & E ×125); (c) showing inflammatory cellular infiltration with presence of pus cells and early collagen fibrous formation with mild oedema (H & E ×125); (d) showing thin epidermis (lost reteridges) with mild inflammatory infiltration (H & E ×125); (e) [example of skin finding in these groups] skin section showing intact epidermis with prominant dermal fibrosis (H & E ×125); (f) showing moderate inflammatory infiltration with mild oedema. [example of skin finding in these groups] (H & E ×125); (g) showing epidermal thickening dermal oedema and fibrosis with mild inflammatory infiltration (H & E ×125); (h) showing dense dermal acute inflammatory cellular infiltration (H & E ×125); (i) Skin section of hind paw of rat showing mild oedematous spaces and dilated engorged capillaries (H & E ×250); (j) showing mild to moderate inflammatory cellular infiltration and oedema (H & E ×125).
4. Discussion
Antibiotics represent the bulwark to combat bacterial infections, but the spread of antibiotic resistance compromises their clinical efficacy [5] .
Increasing multidrug resistance in Gram-negative bacteria, presents a critical problem with increasing threat to human health. Limited therapeutic options have forced infectious disease clinicians and microbiologists to reappraise the clinical application of old antibiotics. Recent clinical findings focus on evaluation of efficacy, potential toxicities and combination therapy [51] [52] . The focus was directed to the clinical aspects such as the efficiency of antibiotics in clearing infections and pathogens that are resistant to antibiotic treatment [53] .
On account of that, it was very interesting to investigate the possible mechanisms beyond the antibacterial activity of some antibiotics that contribute to curing resistant bacterial infection.
The present study focused on assessment of the efficacy of erythromycin and chloramphenicol (narrow spectrum and old antibiotics) in healing E. coli infected wounds. As the shortage in discovering new antibiotics concurrently with wide spread of resistant bacterial infections, paid attention to re-evaluation of old antibiotics using highest safest dose of them or using them in combination with other drugs.
Almost all infectious diseases have a substantial inflammatory component and if left unchecked, the host’s proinflammatory responses may cause more harm than a pathogen would. In this regard, the anti-inflammatory activities of macrolides may help to alleviate the overly aggressive inflammatory responses and prevent the resulting excessive tissue damage [54] . Accordingly this work included in vitro and in vivo studies.
In vitro study includes screening of resistancy of E. coli to tested antibiotics, where it was confirmed that all of these bacterial strains were resistant to tested antibiotics either alone or in combination with tenoxicam, as standard NSAID. The addition of tenoxicam to those antibiotics, has indifferent effect on the MICs of those antibiotics on the examined bacterial strains. The present data found that tenoxicam alone lacked antimicrobial activity against those tested isolates.
Tenoxicam has protective effects on the oxidative stress by inhibiting free radical and supporting antioxidant redox system. It decreased the chemotaxis in human monocytes and polymorphonuclear cells [33] [55] . Therefore tenoxicam can be used as control for assessment of anti-infalmmatory efficacy of tested drugs.
In vivo study has included wound infection in rat hind paw animal model, using selected one of highly MAR strains of E. coli, which revealed that erythromycin was effective in treatment of those wounds despite of resistancy in vitro but chloramphenicol was not effective.
Gram-negative organisms such as E. coli are intrinsically resistant to macrolide antibiotics [56] , some strains of E. coli, show spontaneous emergence of resistance to chloramphenicol [57] [58] . E. coli was used as a model system to explore the genetic basis of intrinsic multidrug resistance which is a major cause of clinical failure in treating bacterial infections. Increasing evidence suggests that bacteria can resist multiple antibiotics through intrinsic mechanisms [59] .
Bacterial LPS, the cell wall component of all gram-negative bacteria including E. coli, causes the systemic inflammatory response syndrome and septic shock, which finally results in multiorgan failure in humans and animal models. LPS triggers the synthesis and release of cytokines, NO, and ROS [60] [61] . Thus inhibition of unresolved inflammation, either by antibiotics or specifically anti-inflammatory agents is needed to relieve patients with chronic inflammatory disorders [13] .
Investigation in the present study of cytokines expression; inflammatory reaction and oxidative stress were considered as possible pathways. It has been revealed significant down regulation of hind paw inflammation, some immunological mediators (Il-1β, TNF-α, NO, MDA) and increased GSH by erythromycin but chloramphnicol treated rats couldn’t be completely healed or had any significant effects on the investigated immunological mediators.
On account of that, it was necessary to confirm obtained results from bacterial infected model by using carrageenan-induced acute inflammation in rats model, that was achieved examining the effects of different concentrations of tested antibiotics on the investigated immunological mediators.
Where carrageenan induced inflammation (paw oedema) animal model was adopted for the quantification of the state of inflammation. It was a useful model to assess the contribution of mediators involved in vascular changes associated with acute inflammation and produce this inflammatory response [62] - [64] .
Hind paw inflammation in this study was evaluated in both E.coli infected rat model and carrageenan-induced paw oedema rat model, the inflammation was significantly reduced by erythromycin but not chloramphenicol.
Cytokines are key modulators of inflammation. They participate in acute and chronic inflammation in a complex network of interactions. Several cytokines exhibit some redundancy in function and share overlapping properties. Better understanding of the pathways regulated by cytokines will allow the identification and/or development of agents for improved modulation of the inflammatory response for the treatment of autoimmune, infectious, and neoplastic diseases [65] .
Il-1β is a potent proinflammatory cytokine that has been implicated in numerous physiological processes as well as inflammatory diseases and appears to have deleterious effects on the host [66] [67] .
IL-1β in E. coli infected animal model of the present study significantly decreased by erythromycin by approximately ninety percent. Similarly, when using combination of erythromycin and tenoxicam, it revealed synergestic effect in vivo irrespective of its indifferent effect in vitro. On the other hand chloramphenicol either alone or in combination with tenoxicam showed insignificant reduction of IL-1β around twenty percent.
IL-1β in carrageenin acute inflammatory model was significantly decreased by erythromycin in a dose dependent manner while chloramphenicol did not significantly decrease it.
Those effects of examined antibiotics on IL-1β were coincided with previous reports where, it was demonstrated that an absence or reduction in endogenous IL-1β activity improves host defense against Pseudomonas pneumonia while suppressing the inflammatory response [67] . Apparently, sustained levels of IL-1β enhanced susceptibility to persistent Staphylococcus epidermidis infection in tissues surrounding implanted biomaterial (catheter) in mice [68] . It was suggested the importance of the host response to various biomaterials in the pathogenesis of biomaterial associated infection (BAI), rather than for the extent of adherence of bacteria to biomaterial. Therefore Local inhibition of IL-1β activity may be of benefit to the host as an adjunctive therapy for infections associated with biomaterials [69] .
Apart from direct antimicrobial effects, macrolides are known for their modulating effect on many components of the human immune system. By influencing the production of cytokines, they have a dampening effect on the proinflammatory response. Having such an obvious effect on the various aspects of the immune system, macrolides seem to be exceptionally suited for the treatment of chronic inflammatory diseases [51] [70] .
Erythromycin mechanisms underlying the therapeutic efficacy in DPB were found to be without affecting the number of viable bacteria recovered from the infected lung, it resulted in a significant reduction of IL-1β in an experimental rabbit model of DPB induced by Ps. aeruginosa inoculation [71] . It was suggested that long-term, low-dose administration of macrolide antibiotics is associated with tissue reparative and anti-inflammatory effects that are beyond their anti-infective properties, in patients with chronic inflammatory sinopulmonary diseases such as chronic sinusitis, asthma, bronchiectasis, cystic fibrosis, and diffuse panbronchiolitis, who do not show evidence of a bacterial infection. Importantly, the prolonged use of these drugs is not associated with emergence of clinically significant bacterial resistance or immunosuppression [72] [73] .
TNF-α is a cytokine that plays a critical role in both acute and chronic inflammation [74] . Mice deficient for the type 1 TNF receptor demonstrated an enhanced early clearance of P. aeruginosa from the lungs during sub- acute pneumonia [75] . TNF-α in E.coli infected animal model of the present study significantly decreased by erythromycin alone and in combination with tenoxicam by approximately ninety percent, which revealed synergestic effect in vivo irrespective indifference in vitro. But chloramphenicol either alone or in combination with tenoxicam showed insignificant reduction of TNF-α.
TNF-α in carrageenin acute inflammatory model significantly decreased by erythromycin in a dose dependent manner while chloramphenicol not significantly decreased it.
Carrageenan-induced acute inflammation in rats causes increase the proinflammatory cytokine TNF-α, that are activated in certain inflammatory conditions [76] - [78] .
Tenoxicam exhibited a pronounced inhibitory effect upon the production of TNF-α in vitro [79] .
Erythromycin caused a dose-dependent decrease in the production of pro-inflammatory cytokines e.g TNF-α, that may, at least in part, explain the efficacy of this macrolide during panbronchiolitis and DPB despite its lack of activity for P. aeruginosa [71] [80] , which is in line with the effect of this antibiotic on TNF-α production induced by Streptococcus pneumoniae [81] [82] . Consquently local modulation of the cytokine network may serve as an important addition to antibiotic therapy [80] .
NO is another key mediator produced in many inflammatory and infectious conditions by inducible NO synthase (iNOS). In mammals including humans, NO is an important cellular signaling molecule involved in many physiological and pathological processes [83] , NO has dual activity on oxidative cell killing with low concentrations protecting against cell death, whereas higher concentrations are cytotoxic [84] . It is potent inflammatory mediator because of its strong reactivity with oxygen, superoxide and iron-containing compounds. IL-1β and TNF-α are involved in the formation of toxic peroxynitrite by increasing the activity of NOS enzyme [85] [86] .
NO in infected animal model of the present study significantly decreased by erythromycin alone and with tenoxicam by approximately seventy percent. This represented synergestic effect in vivo despite of its abscence in vitro. On the other hand chloramphenicol either alone or in combination with tenoxicam showed insignificant reduction of NO.
NO in carrageenan-induced acute inflammatiory model was modulated to reach its normal levels as naïve in a dose dependent manner by cefradine, erythromycin and ampicillin in a dose dependent manner while chloramphenicol not significantly reduced it.
NO appears to be involved in the acute inflammatory response following the intraplantar injection of carrageenan into the rat hind paw [87] . So that one of the anti-inflammatory activities is through inhibition of NO production [88] .
Macrolide antibiotics have anti-inflammatory activity that plays a prominent role in some infections [54] [89] [90] . Macrolides appear to inhibit NO production by blocking NOS expression in rats. As a high level of NO has been associated with inflammation, NO inhibition may be one of multiple anti-inflammatory mechanisms of macrolides [91] [92] . Also tenoxicam-treated patients had a significant decrease in nitrite levels [93] . But chloramphenicol increases nitrite production in neutrophils incubated with a corresponding increase in antibiotic concentration [94] . Thus inhibition of NO production paid attention to the anti-inflammatory activity of erythromycin.
ROS can serve as both intra-and inter-cellular messengers, they have a role in cell signaling, including; apoptosis; gene expression; and the activation of cell signaling cascades [95] [96] . The regulation of ROS production is particularly important in neutrophils, because these cells perform an important function in host defence against bacterial infections by producing ROS and nitrogen species, hydrolytic and proteolytic enzymes, and antimicrobial polypeptides [97] . Deregulation of nitric oxide and increased oxidative and nitrosative stress are implicated in tissue damage [98] . The body on account of susceptibility to oxidative insult, is naturally provided with an efficient antioxidant defense enzymes as scavenging systems including superoxide dismutase (SOD), catalase and glutathione peroxidase and reduced glutathione (GSH). These enzymes are the first line of defense against ROS and are generally referred to as primary antioxidants [99] . Oxidative stress, occurs when ROS overwhelm cellular antioxidant systems, contributes to the pathophysiology of many chronic inflammatory diseases and can alter cellular processes, including signaling pathways, metabolic pathways, transcription, and translation [100] [101] .
GSH is considered to be one of the most important scavengers of ROS [102] . It is the most significant non enzymatic oxidant defense mechanism, it plays an important role in the protection of cells and tissue structures. It exists in relatively large amounts. Its role includes detoxication of free radicals, peroxides, regulation of immune function and regenerate a number of important antioxidants (e.g. α-tocopherol and ascorbic acid) [86] [103] [104] .
GSH in the E. coli infected animal model of the present study significantly increased by erythromycin by approximately three hundreds eighty percent. Similarly, when using combination between this antibiotic and tenoxicam which revealed synergestic activity in vivo irrespective of its indifference in vitro. On the other hand chloramphenicol either alone or in combination with tenoxicam showed insignificant increase of GSH. Similarly GSH in carrageenan-induced acute inflammatiory model was elevated to reach its normal levels as naïve in a dose dependent manner by erythromycin while chloramphenicol not significantly increase it, by using vitamin C as standard antioxidant for assessment of antioxidant activity of tested drugs, where vitamin C may prevent dysregulation of the immune-inflammatory response by its antioxidant properties [105] .
Erythromycin exhibits antioxidant properties and can increase the synthesis of GSH [106] - [108] .
Chloramphenicol has been demonstrated to affect the oxidative state of cellular components by increasing the intracellular ROS, while the glutathione level decreased in neutrophils [94] [109] .
MDA is the end product of LPO that result from ROS attack to polyunsaturated fatty acids in the membrane lipids. Thus assessment of MDA is one of the most commonly applied methods and a sensitive index of lipid peroxidation and oxidative stress [99] - [110] .
MDA in the E. coli infected animal model of the present study significantly decreased by erythromycin alone/ and with tenoxicam showing synergestic effect in vivo. on the other hand chloramphenicol either alone or in combination with tenoxicam showed insignificant decrease of MDA. Similarly in carrageenan-induced acute inflammatiory model, MDA was reduced to reach its normal levels as naïve in a dose dependent manner by erythromycin while chloramphenicol did not significantly reduce it.
Erythromycin inhibits inflammation via downregulation of pro-inflammatory cytokines, chemokines and oxidant production [111] - [114] . Tenoxicam may have antioxidant effects where treated patients had a significant decrease in MDA levels [93] . Carrageenan-induced paw oedema is neutrophil dependent [115] . Where activated neutrophils, are an excellent source of oxygen-derived free radicals which have been implicated in many models of acute inflammation in which there is a neutrophil-dependent increase in vascular permeability [116] . It was reported that the removal of oxygen-derived free radicals significantly inhibited the paw oedema [63] [115] .
Thus Antibiotics interference with the host’s immune cells such as lymphocytes and phagocytes, (immunomodulation) is believed to occur via alterations in chemotaxis, phagocytosis, oxidative burst, complement activation and cytokine secretion [117] [118] .
Histopathological changes have been performed, in confirmation to the obtained previous data including cytokines, antioxidants and other mediators. The infection by E. coli in rat hind paw has shown severe oedema, prominent capillary diltation and prevascular inflammatory cellular infiltration down to the deep dermis with ulceration and pyogenic membrane formation however the induction of inflammation in rat hind paw by carrageenin has shown signs of dense dermal acute inflammatory cellular infiltration that lead to oedema. Both infected and acute inflammatory animal models have revealed that skin section from erythromycin treated groups showing intact epidermis with prominant dermal fibrosis. Tenoxicam treated group in infected model showed decrease of inflammatory reaction signs while in acute inflammatory model tenoxicam or vitamin C treated groups showing apparently normal sections as control group with early inflammatory changes manifested as mild oedema with dilated, congested capillaries and normal covering epidermis. Chloramphenicol shows mild to moderate inflammatory cellular infiltration and oedema.
Concurrently some previous reports are mostly compatible with the present results of healing E. coli infected wounds in vivo by erythromycin, despite lack of activity in vitro, such as Van Bambeke et al. (1996); Anderson et al. (1996), Feldman et al. ( 1997) and Garey et al. (2003), who had reported that long-term, low-dose administration of macrolide antibiotics regulate key intracellular metabolic and transcriptional pathways involved in the inflammatory cascade, such as ROS, NO and cytokines [72] [119] - [121] .
Schultz et al. (2001) have reported that erythromycin but not antibiotics with antimicrobial activity against Pseudomonas spp., is beneficial in panbronchiolitis patients infected with Ps. Aeruginosa [80] .
The reduction in the inflammatory capacity of the colonizing organisms could enhance clinical improvement which suggessted new role for antibiotics as adjunctive therapy in atopic dermatitis and other diseases whose severity is increased by bacterial colonization of the affected areas [122] . The recognition that shed bacterial components efficiently activate pro-inflammatory signaling in epithelial cells may provide novel targets for anti-inflammatory therapy, e.g. the control of airway inflammation through modulation of inflammatory signaling may prove to be an effective mode of therapy to prevent lung injury [123] . In vitro experiments of the four macrolides (roxithromycin, clarithromycin, erythromycin, and azithromycin) distinctly had shown reducion in a concentration-dependent manner of generation of some mediators and cytokines involved in the inflammatory process such as NO, TNF-α, IL-1β by lipopolysaccharide-stimulated macrophages, independently of their antibacterial activity [124] .
So that effects of macrolides on neutrophil cell function and cytokine production and attenuation of biofilm formation may be responsible for the beneficial effects of macrolides in patients with DPB or CF, which are not related to their antimicrobial properties, since levels of macrolides with low-dose treatment are too low to have sufficient antimicrobial effects. This ability of biofilm attenuation, affects isolates that attach to either the natural airways of the patient or the synthetic material used for endotracheal/tracheostomy tubes, and occurs at subminimal inhibitory concentrations of the macrolides [89] [123] [125] - [129] . macrolide lead to improved quality and quantity of life for patients with CF and asthma [130] .
Also from the possible mechanisms which could explain modification of the host cell responses were accumulation of agents such as macrolides that accumulate intracellularly within lymphoid cells or those that interfere with protein or DNA synthesis [118] . In addition Yamasaki (1990) demonstrated a reduction in adherence of P. aeruginosa treated with erythromycin to acid-injured mice tracheal epithelium in vitro [131] .
It seems more likely that bacteria or bacterial components interact with surface epithelial cells to induce an inflammatory response [132] [133] . So that the potential benefits of downregulating immunomodulators entered the limelight, with the understanding that immune hyperactivation (in sepsis and inflammatory/ autoimmune diseases, for example) can also have disastrous consequences. Incidental observations that some non-infectious diseases, including inflammatory disorders, may be improved by antibacterials have bolstered interest in the immunomodulatory activity of this class of drugs [11] .
This pay attention to the growing evidence that certain antibiotics exert their beneficial effects not only by killing or inhibiting the growth of bacterial pathogens but also indirectly by immunomodulation due to immunomodulatory properties of antibiotics in different diseases [51] . Immunomodulatory therapies are defined as interventions that target the host rather than the pathogen, modulating the immune response with the aim of disease prevention or treatment [134] .
The immunomodulatory properties of various antibacterial agents were demonstrated in vitro and in vivo, such as fluoroquinolones and tetracyclines [135] - [138] .
Therfore the knowledge of the effects of antibiotics on the immune response, the synthesis and secretion of pro-inflammatory cytokines allows us to see the drugs known for years in a new light. This includes finding for them new applications beyond the infection treatment such as in organ transplantation, invasive cardiology and treatment of autoimmune diseases e.g rheumatoid arthritis or asthma. Yet, undoubtedly, this line of drug development needs further research [139] .
Immunomodulatory effect of macrolides has been documented in the treatment of rheumatoid arthritis (RA), still’s disease in adults and inflammatory bowel diseases as well as in adjuvant arthritis [139] [140] . Therefore in the battle against rapidly emerging bacterial resistance we can no longer rely entirely on the discovery of new antibiotics; we must also pursue rational approaches to the use of older antibiotics [2] [8] [52] . Thus in vivo studying of antibiotics efficacy in curing resistant bacterial infections is very valuable as it take into consideration the interaction between these antimicrobial agents and immune system of the host.
5. Conclusion
Collectively, we concluded that erythromycin had significant healing efficacy for bacterial infected wounds in rat hind paw, despite of resistancy to it in vitro while chloramphenicol didn’t have this effect. This curing activity could be explained through other possible mechanisms beyond their antibacterial activity, such as immunomodulatory, anti-inflammatory and anti-oxidant activities on rat hind paw inflammation, and investigated immunological mediators (IL-1β; TNF-α; NO, MDA, GSH) released in response to E. coli infection. Also efficacy of erythromycin increased with tenoxicam combination that leading to synergestic effects. Thus it was obvious that some antibiotics could possess interactions with the immune system irrespective of their antibacterial actions which could promote healing of resistant bacterial infections. Consequently this could breathe life in older antimicrobial agents.
References
- Carlet, J., Jarlier, V., Harbarth, S., Voss, A., Goossens, H. and Pittet, D. (2012) Ready for a World without Antibiotics? The Pensières Antibiotic Resistance Call to Action. Antimicrobial Resistance and Infection Control, 1, 11. http://dx.doi.org/10.1186/2047-2994-1-11
- Falagas, M., Bliziotis, I., Kasiakou, S., Samonis, G., Athanassopoulou, P. and Michalopoulos, A. (2005) Outcome of Infections Due to Pandrug-Resistant (PDR) Gram-Negative Bacteria. BMC Infectious Diseases, 5, 24. http://dx.doi.org/10.1186/1471-2334-5-24
- Raz, R. (2012) Fosfomycin: An Old-New Antibiotic. Clinical Microbiology and Infection, 18, 4-7. http://dx.doi.org/10.1111/j.1469-0691.2011.03636.x
- Tsai, W.C., Hershenson, M.B., Zhou, Y. and Sajjan, U. (2009) Azithromycin Increases Survival and Reduces Lung Inflammation in Cystic Fibrosis Mice. Inflammation Research, 58, 491-501. http://dx.doi.org/10.1007/s00011-009-0015-9
- Imperi, F., Leoni, L. and Visca, P. (2014) Antivirulence Activity of Azithromycin in Pseudomonas aeruginosa. Frontiers in Microbiology, 5, 178. http://dx.doi.org/10.3389/fmicb.2014.00178
- Garnacho-Montero, J., Ortiz-Leyba, C., Jimenez-Jimenez, F.J., Barrero-Almodovar, A.E., Garcia-Garmendia, J.L., Bernabeu-WittelI, M., Gallego-Lara, S.L. and Madrazo-Osuna, J. (2003) Treatment of Multidrug-Resistant Acinetobacter baumannii Ventilator-Associated Pneumonia (VAP) with Intravenous Colistin: A Comparison with Imipenem- Susceptible VAP. Clinical Infectious Diseases, 36, 1111-1118. http://dx.doi.org/10.1086/374337
- Michalopoulos, A.S., Tsiodras, S., Rellos, K., Mentzelopoulos, S. and Falagas, M.E. (2005) Colistin Treatment in Patients with ICU-Acquired Infections Caused by Multiresistant Gram-Negative Bacteria: The Renaissance of an Old Antibiotic. Clinical Microbiology and Infection, 11, 115-121. http://dx.doi.org/10.1111/j.1469-0691.2004.01043.x
- Velkov, T., Roberts, K.D., Nation, R.L., Thompson, P.E. and Li, J. (2013) Pharmacology of Polymyxins: New Insights into an “Old” Class of Antibiotics. Future Microbiology, 8, 711-724. http://dx.doi.org/10.2217/fmb.13.39
- Alanis, A.J. (2005) Resistance to Antibiotics: Are We in the Post-Antibiotic Era? Archives of Medical Research, 36, 697-705. http://dx.doi.org/10.1016/j.arcmed.2005.06.009
- Mandell, G.L. and Coleman, E. (2001) Uptake, Transport, and Delivery of Antimicrobial Agents by Human Polymorphonuclear Neutrophils. Antimicrobial Agents and Chemotherapy, 45, 1794-1798. http://dx.doi.org/10.1128/AAC.45.6.1794-1798.2001
- Labro, M.T. (2011) C10 Influence of Antibacterial Drugs on the Immune System. In: Nijkamp, F.P. and Parnham, M.J., Eds., Principles of Immunopharmacology, Springer, Berlin, 473-506. http://dx.doi.org/10.1007/978-3-0346-0136-8_25
- Ryan, K.J. and Ray, C.G., Eds. (2004) Sherris Medical Microbiology. 4th Edition, McGraw-Hill, New York.
- Parnham, M.J. (2005) Antibiotics, Inflammation and Its Resolution: An Overview. In: Rubin, B.K. and Tamaoki, J., Eds., Antibiotics as Anti-Inflammatory and Immunomodulatory Agents. Progress in Inflammation Research, Springer, Berlin, 27-47. http://dx.doi.org/10.1007/3-7643-7310-5_2
- Bhadauria, A.R. and Hariharan, C. (2013) Clinical Study of Post Operative Wound Infections in Obstetrics and Gynaecological Surgeries in a Tertiary Care Set Up. International Journal of Reproduction, Contraception, Obstetrics and Gynecology, 2, 631-638. http://dx.doi.org/10.5455/2320-1770.ijrcog20131228
- Dunne, M., Mason, E.O. and Kaplan, S.L. (1993) Diffusion of Rifampin and Vancomycin through a Staphylococcus epidermidis Biofilm. Antimicrobial Agents and Chemotherapy, 37, 2522-2526.
- Amorena, B., Gracia, E., Monzon, M., Leiva, J., Oteiza, C., Pérez, M., Alabart, J. and Hernández-Yago, J. (1999) Antibiotic Susceptibility Assay for Staphylococcus aureus in Biofilms Developed in Vitro. Journal of Antimicrobial Chemotherapy, 44, 43-55. http://dx.doi.org/10.1093/jac/44.1.43
- Davis, C., Martiner, L. and Kirsner, R. (2006) The Diabetic Foot: The Importance of Biofilms and Wound Bed Preparation. Current Diabetes Reports, 6, 439-445. http://dx.doi.org/10.1007/s11892-006-0076-x
- Abd El-Aziz, A., El-Banna, T., Abo-Kamar, A., Ghazal, A. and Abozahra, R. (2010) In Vitro and in Vivo Activity of Some Antibiotics against Staphylococcal Biofilm and Planktonic Cells Isolated from Diabetic Foot Infections. Journal of American Science, 6.
- El-Banna, T., Abd El-Aziz, A., Abo-Kamar, A., Ghazal, A. and Abozahra, R. (2010) In Vitro Activities of Three Kinds of Antibiotics against Staphylococcal Biofilm and Planktonic Cultures. African Journal of Microbiology Research, 4, 2275-2282.
- Asada, M., Nakagami, G., Minematsu, T., Nagase, T., Akase, T., Huang, L., Yoshimura, K. and Sanada, H. (2012) Novel Models for Bacterial Colonization and Infection of Full-Thickness Wounds in Rats. Wound Repair and Regeneration, 20, 601-610. http://dx.doi.org/10.1111/j.1524-475x.2012.00800.x
- Okusu, H., Ma, D. and Nikaido, H. (1996) AcrAB Efflux Pump Plays a Major Role in the Antibiotic Resistance Phenotype of Escherichia coli Multiple-Antibiotic-Resistance (Mar) Mutants. Journal of Bacteriology, 178, 306-308.
- Nazeer, H.A., Shaik, K.M. and Kolasani, B.P. (2014) Aerobic Bacteriology of Wound Infections with Special Reference to MRSA. Journal of Clinical & Experimental Research, 2, 74-79. http://dx.doi.org/10.5455/jcer.201411
- Collee, J.G., Fraser, A.G., Marmion, B.P. and Simmons, A. (1996) Mackie and MacCartney Practical Medical Microbiology. 14th Edition, Churchill Livingstone, New York.
- Koneman, E., Winn, W., Allen, S., Janda, W., Procop, G., Berger, P.S. and Woods, G. (2006) Chapter 6. Enterobacteriaceae. In: Winn Jr., W.C., Allen, S.D., Janda, W.M., et al., Eds., Koneman’s Color Atlas and Text Book of Diagnostic Microbiology, 6th Edition, Lippincott Williams and Wilkins, Philadelphia, 211-302.
- Tong, J., Liu, Z.-C. and Wang, D.-X. (2011) Azithromycin Acts as an Immunomodulatory Agent to Suppress the Expression of TREM-1 in Bacillus pyocyaneus-Induced Sepsis. Immunology Letters, 138, 137-143. http://dx.doi.org/10.1016/j.imlet.2011.04.001
- CLSI (2010) Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement. NCCLS/CLSI document MI00-S20-U. Clinical and Laboratory Standards Institute, Wayne.
- Cursino, L., Chartone, E.S. and Nascimento, A.M.A. (2005) Synergic Interaction between Ascorbic Acid and Antibiotics against Pseudomonas aeruginosa. Brazilian Archives of Biology and Technology, 4, 3. http://dx.doi.org/10.1590/s1516-89132005000300007
- Paino, I.M.M., Ximenes, V.F., da Fonseca, L.M., Kanegae, M.P.P., Khalil, N.M. and Brunetti, I.L. (2005) Effect of Therapeutic Plasma Concentrations of Non-Steroidal Anti-Inflammatory Drugs on the Production of Reactive Oxygen Species by Activated Rat Neutrophils. Brazilian Journal of Medical and Biological Research, 38, 543-551. http://dx.doi.org/10.1590/s0100-879x2005000400007
- Mackay, M.L., Milne, K. and Gould, I.M. (2000) Comparison of the Methods for Assessing Synergic Antibiotic Interactions. International Journal of Antimicrobial Agents, 15, 125-129. http://dx.doi.org/10.1016/S0924-8579(00)00149-7
- Odds, F.C. (2003) Synergy, Antagonism, and What the Chequerboard Puts between Them. Journal of Antimicrobial Chemotherapy, 52, 1. http://dx.doi.org/10.1093/jac/dkg301
- Lau, T.W., Lam, F.F.Y., Lau, K.M., Chan, Y.W., Lee, K.M., Sahota, D.S., Ho, Y.Y., Fung, K.P., Leung, P.C. and Lau, C.B.S. (2009) Pharmacological Investigation on the Wound Healing Effects of Radix Rehmanniae in an Animal Model of Diabetic Foot Ulcer. Journal of Ethnopharmacology, 123, 155-162. http://dx.doi.org/10.1016/j.jep.2009.02.010
- Davis, S.C., Ricotti, C., Cazzaniga, A., Welsh, E., Eaglstein, W.H. and Mertz, P.M. (2008) Microscopic and Physiologic Evidence for Biofilm-Associated Wound Colonization in Vivo. Wound Repair and Regeneration, 16, 23-29. http://dx.doi.org/10.1111/j.1524-475X.2007.00303.x
- Naziroğlu, M., Uğuz, AC., Gokçimen, A., Bülbül, M., Karatopuk, DU., Türker, Y. and Cerçi, C. (2008) Tenoxicam Modulates Antioxidant Redox System and Lipid Peroxidation in Rat Brain. Neurochemical Research, 33, 1832-1837. http://dx.doi.org/10.1007/s11064-008-9643-7
- Amacher, D.E., Schomaker, S.J. and Retsema, J.A. (1991) Comparison of the Effects of the New Azalide Antibiotic, Azithromycin and Erythromycin Estolate on Rat Liver Cytochrome P-450. Antimicrobial Agents and Chemotherapy, 35, 1186-1190. http://dx.doi.org/10.1128/AAC.35.6.1186
- Halpert, J., Balfour, C., Miller, N.E., Morgan, E.T., Dunbar, D. and Kaminsky, L.S. (1985) Isozyme Selectivity of the Inhibition of Rat Liver Cytochromes P-450 by Chloramphenicol in Vivo. Molecular Pharmacology, 28, 290-296.
- Lau, T.W., Sahota, D.S., Lau, C.H., Chan, C.M., Lam, F.C., Ho, Y.Y., Fung, K.P., Lau, C.B.S. and Leung, P.C. (2008) An in Vivo Investigation on the Wound Healing Effect of Two Medicinal Herbs Using an Animal Model with Foot Ulcer. European Surgical Research, 41, 15-23. http://dx.doi.org/10.1159/000122834
- Tasleem, F., Azhar, I., Ali, S.N., Perveen, S. and Mahmood, Z.A. (2014) Analgesic and Anti-Inflammatory Activities of Piper nigrum L. Asian Pacific Journal of Tropical Medicine, 7, S461-S468. http://dx.doi.org/10.1016/S1995-7645(14)60275-3
- Wang, W., Wang, S.-X. and Guan, H.-S. (2012) The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Marine Drugs, 10, 2795-2816. http://dx.doi.org/10.3390/md10122795
- Radhakrishnan, R., Moore, S.A. and Sluka, K.A. (2003) Unilateral Carrageenan Injection into Muscle or Joint Induces Chronic Bilateral Hyperalgesia in Rats. Pain, 104, 567-77. http://dx.doi.org/10.1016/S0304-3959(03)00114-3
- Winter, C.A., Risley, E.A. and Nuss, G.W. (1962) Carrageenin Induced Edema in Hind Paw on the Rat as an Assay for Antiinflammatory Drugs. Proceedings of the Society for Experimental Biology and Medicine, 111, 544-547. http://dx.doi.org/10.3181/00379727-111-27849
- Fath, R.B, Deschner, E.E., Winawer, S.J. and Dworkin, B.M. (1984) Degraded Carrageenan-Induced Colitis in CF1 Mice. A Clinical, Histopathological and Kinetic Analysis. Digestion, 29, 197-203. http://dx.doi.org/10.1159/000199033
- Al-Arfaj, A.S., Mustafa, A.A., Alballa, S.R., Tuwaijri, A.S. and Al-Dalaan, A.N. (2003) Interaction of Allopurinol and Non-Steroidal Anti-Inflammatory Drugs on the Carrageenan-Induced Rat Paw Edema. Saudi Medical Journal, 24, 936-940.
- Igbal, K., Khan, A. and Khattak, M.M.A.K. (2004) Biological Significance of Ascorbic Acid (Vitamin C) in Human Health―A Review. Pakistan Journal of Nutrition, 3, 5-13. http://dx.doi.org/10.3923/pjn.2004.5.13
- Ikeda, M., Nakabayashi, K., Shinkai, M., Hara, Y., Kizaki, T., Oh-ishi, S. and Ohno, H. (2004) Supplementation of Antioxidants Prevents Oxidative Stress during a Deep Saturation Dive. The Tohoku Journal of Experimental Medicine, 203, 353-357. http://dx.doi.org/10.1620/tjem.203.353
- Kanter, M., Coskun, O., Armutcu, F., Uz, Y.H. and Kizilay, G. (2005) Protective Effects of Vitamin C, Alone or in Combination with Vitamin A, on Endotoxin-Induced Oxidative Renal Tissue Damage in Rats. The Tohoku Journal of Experimental Medicine, 206, 155-162. http://dx.doi.org/10.1620/tjem.206.155
- Miranda, K., Espey, M. and Wink, D. (2001) A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide, 5, 62-71. http://dx.doi.org/10.1006/niox.2000.0319
- Ellman, G.L. (1959) Tissue Sulfahydryl Groups. Archives of Biochemistry and Biophysics, 82, 70-77. http://dx.doi.org/10.1016/0003-9861(59)90090-6
- Yoshioka, T., Kawada, K., Shimada, T. and Mori, M. (1979) Lipid Peroxidation in Maternal and Cord Blood and Protective Mechanism against Activated-Oxygen Toxicity in Blood. American Journal of Obstetrics Gynecology, 135, 372-376.
- Bancroft, J.D. and Stevens, A. (1975) Histopathological Stains and Their Diagnostic Uses. Churchill Livingstone, Edinburgh, London and New York.
- Kugelberg, E., Norstrom, T., Petersen, T. K., Petersen, T.K. and Duvold, T. (2005) Establishment of a Superfacial Skin Infection Model in Mice by Using Staphylococcus aureus and Streptococcus pyogens. Antimicrobial Agents and Chemotherapy, 49, 3435-3441. http://dx.doi.org/10.1128/AAC.49.8.3435-3441.2005
- Nau, R. and Tauber, S.C. (2008) Immunomodulatory Properties of Antibiotics. Current Molecular Pharmacology, 1, 68-79. http://dx.doi.org/10.2174/1874467210801010068
- Li, J., Nation, R.L., Turnidge, J.D., Milne, R.W., Coulthard, K., Rayner, C.R. and Paterson, D.L. (2006) Colistin: The Re-Emerging Antibiotic for Multidrug-Resistant Gram-Negative Bacterial Infections. The Lancet Infectious Diseases, 6, 589-601. http://dx.doi.org/10.1016/S1473-3099(06)70580-1
- Aminov, R.I. (2009) The Role of Antibiotics and Antibiotic Resistance in Nature. Environmental Microbiology, 11, 2970-2988. http://dx.doi.org/10.1111/j.1462-2920.2009.01972.x
- Aminov, R.I. (2013) Biotic Acts of Antibiotics. Frontiers in Microbiology, 4, 241. http://dx.doi.org/10.3389/fmicb.2013.00241
- Sacerdote, P. and Panerai, A.E. (1993) Effect of Tenoxicam and Indomethacin on the Chemotaxis Induced by Substance P and Interleukin-8 on Human Monocytes and Polymorphonuclear Cells. International Journal of Tissue Reactions, 15, 175-180.
- Chollet, R., Chevalier, J., Bryskier, A. and Pages, J.M. (2004) The AcrAB-TolC Pump Is Involved in Macrolide Resistance but Not in Telithromycin Efflux in Enterobacter aerogenes and Escherichia coli. Antimicrobial Agents and Chemotherapy, 48, 3621-3624. http://dx.doi.org/10.1128/AAC.48.9.3621-3624.2004
- Tuomanen, E. (1986) Phenotypic Tolerance: The Search for β-Lactam Antibiotics That Kill Nongrowing Bacteria. Review of Infectious Diseases, 8, S279-S291. http://dx.doi.org/10.1093/clinids/8.supplement_3.s279
- Carone, B.R., Xu, T., Murphy, K.C. and Marinus, M.G. (2014) High Incidence of Multiple Antibiotic Resistant Cells in Cultures of in Enterohemorrhagic Escherichia coli O157:H7. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 759, 1-8. http://dx.doi.org/10.1016/j.mrfmmm.2013.11.008
- Duo, M., Hou, S. and Ren, D. (2008) Identifying Escherichia coli Genes Involved in Intrinsic Multidrug Resistance. Applied Microbiology and Biotechnology, 81, 731-41. http://dx.doi.org/10.1007/s00253-008-1709-6
- Zhang, C., Walker, L.M. and Mayeux, P.R. (2000) Role of Nitric Oxide in Lipopolysaccharide-Induced Oxidant Stress in the Rat Kidney. Biochemical Pharmacology, 59, 203-209. http://dx.doi.org/10.1016/s0006-2952(99)00324-x
- Cimen, B., Turkozkan, N., Unlu, A. and Karasu, C. (2005) Effects of Melatonin on 3-Nitrotyrosine Formation and Energy Charge Ratio in Guinea Pig Kidney in LPS-Induced Stress. Cell Biochemistry and Function, 23, 273-277. http://dx.doi.org/10.1002/cbf.1151
- Vinegar, R., Schreiber, W. and Hugo, R. (1969) Biophasic Development of Carrageenan Odema in Rats. Journal of Pharmacology and Experimental Therapeutics, 166, 96-103.
- Salvemini, D., Wang, Z.Q., Wyatt, P.S., Bourdon, D.M., Marino, M.H., Manning, P.T. and Currie, M.G. (1996) Nitric Oxide: A Key Mediator in the Early and Late Phase of Carrageenan-Induced Rat Paw Inflammation. British Journal of Pharmacology, 118, 829-838. http://dx.doi.org/10.1111/j.1476-5381.1996.tb15475.x
- Lam, F.F.Y. and Ng, E.S.K. (2003) Characterization of Somatostatin Actions on Knee Joint Blood Vessels of the Rat. European Journal of Pharmacology, 474, 295-301. http://dx.doi.org/10.1016/S0014-2999(03)02107-1
- Feghali, C.A. and Wright, T.M. (1997) Cytokines in Acute and Chronic Inflammation. Frontiers in Bioscience, 2, d12-d26.
- Dinarello, CA. (1996) Biologic Basis for Interleukin-1 in Disease. Blood, 87, 2095-2147.
- Schultz, M.J., Rijneveld, W., Floruin, S., Edwards, CK., Dinarello, CA. and Van der Pol, T. (2002) Role of Interleukin-1 in the Pulmonary Immune Responseduring Pseudomonas aeruginosa Pneumonia. American Journal of Physiology-Lung Cellular and Molecular Physiology, 282, L285-L290. http://dx.doi.org/10.1152/ajplung.00461.2000
- Boelens, J.J., Zaat, S.A.J., Murk, J.L., Weening, J.J., Poll, T.V.D. and Dankert, J. (2000) Enhanced Susceptibility to Subcutaneous Abscess Formation and Persistent Infection around Catheters Is Associated with Sustained Interleukin-1β Levels. Infection and Immunity, 68, 1692-1695. http://dx.doi.org/10.1128/IAI.68.3.1692-1695.2000
- Boelens, J.J., Poll, T.V.D., Zaat, S.A.J., Murk, J.L.A.N., Weening, J.J. and Dankert, J. (2000) Interleukin-1 Receptor Type I Gene-Deficient Mice Are Less Susceptible to Staphylococcus epidermidis Biomaterial-Associated Infection than Are Wild-Type Mice. Infection and Immunity, 68, 6924-6931. http://dx.doi.org/10.1128/IAI.68.12.6924-6931.2000
- Altenburg, J., de Graaff, C.S., van der Werf, T.S. and Boersma, W.G. (2011) Immunomodulatory Effects of Macrolide Antibiotics―Part 1: Biological Mechanisms. Respiration, 81, 67-74. http://dx.doi.org/10.1159/000320319
- Takahashi, T., Suga, M., Matsukawa, A., Sato, K., Okamoto, T., Ichiyasu, H., Ohkawara, S., Yoshinaga, M. and Ando, M. (2001) Erythromycin Attenuates an Experimental Model of Chronic Bronchiolitis via Augmenting Monocyte Chemoattractant Protein-1. European Respiratory Journal, 17, 360-367. http://dx.doi.org/10.1183/09031936.01.17303600
- Garey, K.W., Alwani, A., Danziger, L.H. and Rubinstein, I. (2003) Tissue Reparative Effects of Macrolide Antibiotics in Chronic Inflammatory Sinopulmonary Diseases. Chest, 123, 261-265. http://dx.doi.org/10.1378/chest.123.1.261
- Kourlas, H. (2006) Anti-Inflammatory Properties of Macrolide Antibiotics. Journal of Pharmacy Practice, 19, 326-329. http://dx.doi.org/10.1177/0897190006295800
- Holtmann, M.H. and Neurath, M.F. (2004) Differential TNF-Signaling in Chronic Inflammatory Disorders. Current Molecular Medicine, 4, 439-444. http://dx.doi.org/10.2174/1566524043360636
- Skerrett, S.J., Martin, T.R., Chi, E.Y., Peschon, J.J., Mohler, K.M. and Wilson, C.B. (1999) Role of the Type 1 TNF Receptor in Lung Inflammation after Inhalation of Endotoxin or Pseudomonas aeruginosa. American Journal of Physiology-Lung Cellular and Molecular Physiology, 276, L715-L727.
- Nair, M.P., Mahajan, S., Reynolds, J.L., Aalinkeel, R., Nair, H., Schwartz, S.A. and Kandaswami, C. (2006) The Flavonoid Quercetininhibits Proinflammatory Cytokine (Tumor Necrosisfactor Alpha) Gene Expression in Normal Peripheral Mononuclear Cells via Modulation of the NF-κB System. Clinical and Vaccine Immunology, 13, 319-328. http://dx.doi.org/10.1128/CVI.13.3.319-328.2006
- González, R., Ballester, I., López-Posadas, R., Suárez, M.D., Zarzuelo, A., Martínez, O. and Sánchez De Medina, F. (2011) Effects of Flavonoidsand Other Polyphenols on Inflammation. Critical Reviews in Food Science and Nutrition, 51, 331-362. http://dx.doi.org/10.1080/10408390903584094
- Katsori, A.M., Chatzopoulou, M., Dimas, K., Kontogiorgis, C., Patsilinakos, A., Trangas, T. and Hadjipavlou-Litina, D. (2011) Curcuminanalogues as Possible Anti-Proliferative & Anti-Inflammatoryagents. European Journal of Medicinal Chemistry, 46, 2722-2735. http://dx.doi.org/10.1016/j.ejmech.2011.03.060
- Syggelos, S.A., Giannopoulou, E., Gouvousis, P.A., Andonopoulos, A.P., Aletras, A.J. and Panagiotopoulos, E. (2007) In Vitro Effects of Non-Steroidal Anti-Inflammatory Drugs on Cytokine, Prostanoid and Matrix Metalloproteinase Production by Interface Membranes from Loose Hip or Knee Endoprostheses. Osteoarthritis Cartilage, 15, 531-542. http://dx.doi.org/10.1016/j.joca.2006.11.003
- Schultz, M.J., Speelman, P. and van der Poll, T. (2001) Erythromycin Inhibits Pseudomonas aeruginosa-Induced Tumour Necrosis Factor-α Production in Human Whole Blood. Journal of Antimicrobial Chemotherapy, 48, 275-278. http://dx.doi.org/10.1093/jac/48.2.275
- Schultz, M.J., Speelman, P., Zaat, S., van Deventer, S.J.H. and van der Poll, T. (1998) Erythromycin Inhibits Tumor Necrosis Factor Alpha and Interleukin 6 Production Induced by Heat-Killed Streptococcus pneumoniae in Whole Blood. Antimicrobial Agents and Chemotherapy, 42, 1605-1609.
- Schultz, M.J., Speelman, P., Hack, C.E., Buurman, W.A., van Deventer, S.J. and van Der Poll, T. (2000) Intravenous Infusion of Erythromycin Inhibits CXC Chemokine Production, but Augments Neutrophil Degranulation in Whole Blood Stimulated with Streptococcus pneumoniae. Journal of Antimicrobial Chemotherapy, 46, 235-240. http://dx.doi.org/10.1093/jac/46.2.235
- Hou, Y.C., Janczuk, A. and Wang, P.G. (1999) Current Trends in the Development of Nitric Oxide Donors. Current Pharmaceutical Design, 5, 417-441.
- Joshi, M.S., Ponthier, J.L. and Lancaster, J.R. (1999) Cellular Antioxidant and Pro-Oxidant Actions of Nitric Oxide. Free Radical Biology & Medicine, 27, 1357-1366. http://dx.doi.org/10.1016/S0891-5849(99)00179-3
- Ozkan, Y., Yardym-Akaydyn, S., Sepici, A., Keskin, E., Sepici, V. and Simsek, B. (2007) Oxidative Status in Rheumatoid Arthritis. Clinical Rheumatology, 26, 64-68. http://dx.doi.org/10.1007/s10067-006-0244-z
- Meki, A.M.A, Hamed, E.A. and Ezam, K.A. (2009) Effect of Green Tea Extract and Vitamin C on Oxidant or Antioxidant Status of Rheumatoid Arthritis Rat Model. Indian Journal of Clinical Biochemistry, 24, 280-287. http://dx.doi.org/10.1007/s12291-009-0053-7
- Ialenti, A., Ianaro, A., Moncada, S. and Di Rosa, M. (1992) Modulation of Acute Inflammation by Endogenous Nitricoxide. European Journal of Pharmacology, 211, 177-182. http://dx.doi.org/10.1016/0014-2999(92)90526-A
- Maruyama, H., Sakamoto, T., Araki, Y. and Hara, H. (2010) Anti-Inflammatory Effect of Bee Pollen Ethanol Extract from Cistus sp. of Spanish on Carrageenan-Induced Rat Hind Paw Edema. BMC Complementary and Alternative Medicine, 10, 30. http://dx.doi.org/10.1186/1472-6882-10-30
- Amsden, G.W. (2005) Anti-Inflammatory Effects of Macrolides―An Underappreciated Benefit in the Treatment of Community-Acquired Respiratory Tract Infections and Chronic Inflammatory Pulmonary Conditions? Journal of Antimicrobial Chemotherapy, 55, 10-21. http://dx.doi.org/10.1093/jac/dkh519
- Kanoh, S. and Rubin, B.K. (2010) Mechanisms of Action and Clinical Application of Macrolides as Immunomodulatory Medications. Clinical Microbiology Reviews, 23, 590-615. http://dx.doi.org/10.1128/CMR.00078-09
- Kohri, K., Tamaoki, J., Kondo, M., Aoshiba, K., Tagaya, E. and Nagai, A. (2000) Macrolide Antibiotics Inhibit Nitric Oxide Generation by Rat Pulmonary Alveolar Macrophages. European Respiratory Journal, 15, 62-67. http://dx.doi.org/10.1183/09031936.00.15106200
- Tamaoki, J. (2004) The Effects of Macrolides on Inflammatory Cells. Chest, 125, 41-51. http://dx.doi.org/10.1378/chest.125.2_suppl.41S
- Ozgocmen, S., Ardicoglu, O., Erdogan, H., Fadillioglu, E. and Gudul, H. (2005) In Vivo Effect of Celecoxib and Tenoxicam on Oxidant/Anti-Oxidant Status of Patients with Knee Osteoarthritis. Annals of Clinical & Laboratory Science, 35, 137-143.
- Páez, P.L., Becerra, M.C. and Albesa, I. (2008) Chloramphenicol-Induced Oxidative Stress in Human Neutrophils. Basic & Clinical Pharmacology & Toxicology, 103, 349-353. http://dx.doi.org/10.1111/j.1742-7843.2008.00290.x
- Babior, B.M., Kipnes, R.S. and Curnutte, J.T. (1973) Biological Defense Mechanisms. The Productions by Leukocytes of Superoxide, a Potential Bactericidal Agent. Journal of Clinical Investigation, 52, 741-744. http://dx.doi.org/10.1172/JCI107236
- Hancock, J.T., Desikan, R. and Neill, S.J. (2001) Role of Reactive Oxygen Species in Cell Signalling Pathways. Biochemical Society Transactions, 29, 345-350. http://dx.doi.org/10.1042/BST0290345
- Misso, N.L., Peacock, C.D., Watkins, D.N. and Thompson, P.J. (2000) Nitrite Generation and Antioxidant Effects during Neutrophil Apoptosis. Free Radical Biology & Medicine, 28, 934-943. http://dx.doi.org/10.1016/S0891-5849(00)00177-5
- Pacher, P., Schulz, R., Liaudet, L. and Szabó, C. (2005) Nitrosative Stress and Pharmacological Modulation of Heart Failure. Trends in Pharmacological Sciences, 26, 302-310. http://dx.doi.org/10.1016/j.tips.2005.04.003
- Gupta, P., Narang, M., Banerjee, B.D. and Basu, S. (2004) Oxidative Stress in Term Small for Gestational Age Neonates Born to Undernourished Mothers: A Case Control Study. BMC Pediatrics, 4, 14. http://dx.doi.org/10.1186/1471-2431-4-14
- Rice-Evans, C. and Burdon, R. (1993) Free Radical Lipid Interactions and Their Pathological Consequences. Progress in Lipid Research, 32, 71-110. http://dx.doi.org/10.1016/0163-7827(93)90006-I
- Ullevig, S., Kim, H.S. and Asmis, R. (2013) S-Glutathionylation in Monocyte and Macrophage (Dys) Function. International Journal of Molecular Sciences, 14, 15212-15232. http://dx.doi.org/10.3390/ijms140815212
- Zitka, O., Skalickova, S., Gumulec, J., Masarik, M., Adam, V., Hubalek, J., Trnkova, L., Kruseova, J., Eckschlager, T. and Kizek, R. (2012) Redox Status Expressed as GSH:GSSG Ratio as a Marker for Oxidative Stress in Paediatrictumour Patients. Oncology Letters, 4, 1247-1253.
- Ganesan, N., Chegu, H. and Chandrasekaran, A.N. (2003) Effect of Type II Collagen Treatment on the Antioxidant Status in Immune Tissues of Adjuvant Induced Arthritic Rats. Indian Journal of Clinical Biochemistry, 18, 216-22. http://dx.doi.org/10.1007/bf02867390
- Tarpley, M.M., Wink, D.A. and Grisham, M.B. (2004) Methods for Detection of Reactive Metabolites of Oxygen and Nitrogen: In Vitro and in Vivo Considerations. American Journal of Physiology-Regulatory, Integrative and Comparative physiology, 286, R431-R444. http://dx.doi.org/10.1152/ajpregu.00361.2003
- Hartel, C., Strunk, T., Bucsky, P. and Schultz, C. (2004) Effects of Vitamin C on Intracytoplasmic Cytokine Production in Human Whole Blood Monocytes and Lymphocytes. Cytokine, 27, 101-106. http://dx.doi.org/10.1016/j.cyto.2004.02.004
- Hand, W.L., Hand, D.L. and King-Thompson, N.L. (1990) Antibiotic Inhibition of the Respiratory Burst Response in Human Polymorphonuclear Leukocytes. Antimicrobial Agents and Chemotherapy, 34, 863-870. http://dx.doi.org/10.1128/aac.34.5.863
- Sato, E., Nelson, D.K., Koyama, J., Hoyt, J.C. and Robbins, R.A. (2000) Erythromycin Modulates Eosinophil Chemotactic Cytokine Production by Lung Fibroblasts in Vitro. Antimicrobial Agents and Chemotherapy, 45, 401-406. http://dx.doi.org/10.1128/AAC.8.2.401-406.2001
- He, Z.Y., Zou, Z.X., Yu, L., Zhong, N.S., Ran, P.X. and Zhong, X.N. (2005) Effects of Erythromycin on Hydrogen Peroxide-Induced Interleukin-8 Synthesis and Regulation of Glutathione in Human Bronchial Epithelial Cells. Zhonghua Yi Xue Za Zhi (National Medical Journal of China), 85, 976-980.
- Farombi, E.O., Adaramoye, O.A. and Emerole, G.O. (2002) Influence of Chloramphenicol on Rat Hepatic Microsomal Components and Biomarkers of Oxidative Stress: Protective Role of Antioxidants. Pharmacology & Toxicology, 91, 129-134. http://dx.doi.org/10.1034/j.1600-0773.2002.910307.x
- Henrotin, Y.E., Bruckner, P. and Pujol, J.P.L. (2003) The Role of Reactive Oxygen Species in Homeostasis and Degradation of Cartilage. Osteoarthritis and Cartilage, 11, 747-755. http://dx.doi.org/10.1016/S1063-4584(03)00150-X
- Mitsuyama, T., Tanaka, T., Hidaka, K., Abe, M. and Hara, N. (1995) Inhibition by Erythromycin of Superoxide Anion Production by Human Polymorphonuclear Leukocytes through the Action of Cyclic AMP-Dependent Protein Kinase. Respiration, 62, 269-73. http://dx.doi.org/10.1159/000196461
- Wenisch, C., Parschalk, B., Zedtwitz-Liebenstein, K., Weihs, A., el Menyawi, I. and Graninger, W. (1996) Effect of Single Oral Dose of Azithromycin, Clarithromycin, and Roxithromycin on Polymorphonuclear Leukocyte Function Assessed ex Vivo by Flow Cytometry. Antimicrobial Agents and Chemotherapy, 40, 2039-2042.
- Sugiyama, Y., Yanagisawa, K., Tominaga, S.I. and Kitamura, S. (1999) Effects of Long-Term Administration of Erythromycin on Cytokine Production in Rat Alveolar Macrophages. European Respiratory Journal, 14, 113-116. http://dx.doi.org/10.1183/09031936.99.14511139
- Baik, A.R. and Lee, J. (2007) Erythromycin Inhibits Interleukin-6 and Interleukin-8 Expression and Promotes Apoptosis of Activated Human Neutrophils in Vitro. Korean Journal of Physiology & Pharmacology, 11, 259-262.
- Boughton-Smith, N.K., Deakin, A.M., Follenfant, R.L., Whittle, B.J.R. and Garland, L.G. (1993) Role of Oxygenradicals and Arachidonic Acid Metabolites in the Reverse Passive Arthus Reaction and Carrageenin Paw Oedema in the Rat. British Journal of Pharmacology, 110, 896-902. http://dx.doi.org/10.1111/j.1476-5381.1993.tb13897.x
- Fantone, J.C. and Ward, P.A. (1982) Role of Oxygen-Derived Free Radicals and Metabolites in Leukocyte-Dependent Inflammatory Reactions. The American Journal of Pathology, 107, 397-418.
- Labro, M.T. (2000) Interference of Antibacterial Agents with Phagocyte Functions: Immunomodulation or “Immuno-Fairy” Tales? Clinical Microbiology Reviews, 13, 615-650. http://dx.doi.org/10.1128/CMR.13.4.615-650.2000
- Forsgren, A. and Riesbeck, K. (2003) Antibiotics and the Immune System. In: Finch, R.G., Greenwood, D., Norrby, S.R. and Whitley, R.J., Eds., Antibiotic and Chemotherapy, 8th Edition, Churchill Livingstone, London, 101-106.
- Van Bambeke, F., Montenez, J.P., Piret, J., Tulkens, P.M., Courtoy, P.J. and Mingeot-Leclercq, M.P. (1996) Interaction of the Macrolide Azithromycin with Phospholipids: I. Inhibition of Lysosomal Phospholipase A1 Activity. European Journal of Pharmacology, 314, 203-214. http://dx.doi.org/10.1016/S0014-2999(96)00552-3
- Anderson, R., Theron, A.J. and Feldman, C. (1996) Membrane-Stabilizing, Anti-Inflammatory Interactions of Macrolides with Human Neutrophils. Inflammation, 20, 693-705. http://dx.doi.org/10.1007/BF01488805
- Feldman, C., Anderson, R., Theron, A.J., Ramafi, G., Cole, P.J. and Wilson, R. (1997) Roxithromycin, Clarithromycin, and Azithromycin Attenuate the Injurious Effects of Bioactive Phospholipids on Human Respiratory Epithelium in Vitro. Inflammation, 21, 655-665. http://dx.doi.org/10.1023/A:1027342424205
- Jain, A., Sangal, L., Basal, E., Kaushal, G.P. and Agarwal, S.K. (2002) Anti-Inflammatory Effects of Erythromycin and Tetracycline on Propionibacterium acnes Induced Production of Chemotactic Factors and Reactive Oxygen Species by Human Neutrophils. Dermatology Online Journal, 8, 2.
- Schultz, M.J. (2004) Macrolide Activities Beyond Their Antimicrobial Effects: Macrolides in Diffuse Panbronchiolitis and Cystic Fibrosis. Journal of Antimicrobial Chemotherapy, 54, 21-28. http://dx.doi.org/10.1093/jac/dkh309
- Ianaro, A., Ialenti, A., Maffia, P., Sautebin, L., Rombola, L., Carnuccio, R., Iuvone, T., D’Acquisto, F. and Di Rosa, M. (2000) Anti-Inflammatory Activity of Macrolide Antibiotics. The Journal of Pharmacology and Experimental Therapeutics, 292, 156-163.
- Ichimiya, T., Yamasaki, T. and Nasu, M. (1994) In-Vitro Effects of Antimicrobial Agents on Pseudomonas aeruginosa Biofilm Formation. Journal of Antimicrobial Chemotherapy, 34, 331-341. http://dx.doi.org/10.1093/jac/34.3.331
- Takeoka, K., Ichimiya, T., Yamasaki, T. and Nasu, M. (1998) The in Vitro Effect of Macrolides on the Interaction of Human Polymorphonuclear Leukocytes with Pseudomonas aeruginosa in Biofilm. Chemotherapy, 44, 190-197. http://dx.doi.org/10.1159/000007114
- Bui, K.Q., Banevicius, M.A., Nightingale, C.H., Quintiliani, R. and Nicolau, D.P. (2000) In Vitro and in Vivo Influence of Adjunct Clarithromycin on the Treatment of Mucoid Pseudomonas aeruginosa. Journal of Antimicrobial Chemotherapy, 45, 57-62. http://dx.doi.org/10.1093/jac/45.1.57
- Vranes, J. (2000) Effect of Subminimal Inhibitory Concentrations of Azithromycin on Adherence of Pseudomonas aeruginosa to Polystyrene. Journal of Chemotherapy, 12, 280-285. http://dx.doi.org/10.1179/joc.2000.12.4.280
- Yanagihara, K., Tomono, K., Kuroki, M., Kaneko, Y., Sawai, T., Ohno, H., Miyazaki, Y., Higashiyama, Y., Maesaki, S., Kadota, J.I. and Kohno, S. (2000) Intrapulmonary Concentrations of Inflammatory Cytokines in a Mouse Model of Chronic Respiratory Infection Caused by Pseudomonas aeruginosa. Clinical Experimental Immunology, 122, 67-71. http://dx.doi.org/10.1046/j.1365-2249.2000.01343.x
- Sharma, S., Jaffe, A. and Dixon, G. (2007) Immunomodulatory Effects of Macrolide Antibiotics in Respiratory Disease: Therapeutic Implications for Asthma and Cystic Fibrosis. Paediatr. Drugs, 9, 107-118. http://dx.doi.org/10.2165/00148581-200709020-00004
- Yamasaki, T. (1990) Adherence of Pseudomonas aeruginosa to Mouse Tracheal Epithelium: The Effect of Antimicrobial Agents Bronchiolitis. The Japanese Association for Infectious Diseases, 64, 575-583. http://dx.doi.org/10.11150/kansenshogakuzasshi1970.64.575
- Swidsinski, A., Ladhoff, A., Pernthaler, A., Swidsinski, S., Loening-Baucke, V., Ortner, M., Weber, J., Hoffmann, U., Schreiber, S., Dietel, M. and Lochs, H. (2002) Mucosal Flora in Inflammatory Bowel Disease. Gastroenterology, 122, 44-54. http://dx.doi.org/10.1053/gast.2002.30294
- Kotlowski, R., Bernstein, C.N., Sepehri, S. and Krause, D.O. (2007) High Prevalence of Escherichia coli Belonging to the B2+D Phylogenetic Group in Inflammatory Bowel Disease. Gut, 56, 669-675. http://dx.doi.org/10.1136/gut.2006.099796
- Nijnik, A. (2013) Immunomodulatory Approaches for Prevention and Treatment of Infectious Diseases. Current Opinion in Microbiology, 16, 590-595. http://dx.doi.org/10.1016/j.mib.2013.06.011
- Choi, J.H., Song, M.J., Kim, S.H., Choi, S.M., Lee, D.G., Yoo, J.H. and Shin, W.S. (2003) Effect of Moxifloxacin on Production of Proinflammatory Cytokines from Human Peripheral Blood Mononuclear Cells. Antimicrobial Agents and Chemotherapy, 47, 3704-3707. http://dx.doi.org/10.1128/AAC.47.12.3704-3707.2003
- Wise, R.D. (2007) Submicrobial Doxycycline and Rosacea. Comprehensive Therapy, 33, 78-81. http://dx.doi.org/10.1007/s12019-007-8003-x
- Abelson, M.B., Shapiro, A., Makino, A. and Lane, K. (2008) The Other Side of Antibiotics. Review of Ophthalmology, 15, 70-73.
- Labro, M.T. (2012) Immunomodulatory Effects of Antimicrobial Agents. Part I: Antibacterial and Antiviral Agents. Expert Review of Anti-Infective Therapy, 10, 319-340. http://dx.doi.org/10.1586/eri.12.11
- Kwiatkowska, B., Maslinska, M., Przygodzka, M., Dmowska-Chalaba, J., Dabrowska, J. and Sikorska-Siudek, K. (2013) Immune System as a New Therapeutic Target for Antibiotics. Advances in Bioscience and Biotechnology, 4, 91-101. http://dx.doi.org/10.4236/abb.2013.44A013
- Ogrendik, M. (2009) Efficacy of Roxitromycin in Adult Patients with Rheumatoid Arthritis Who Had Not Received Disease-Modifying Antirheumatic Drugs: A 3-Month, Randomized, Double-Blind, Placebo-Controlled Trial. Clinical Therapeutics, 31, 1754-1764. http://dx.doi.org/10.1016/j.clinthera.2009.08.014
NOTES
*Corresponding author.