American Journal of Plant Sciences
Vol.08 No.08(2017), Article ID:77448,19 pages
10.4236/ajps.2017.88123
Callogenesis of Cork Oak (Quercus suber L.) through In Vitro Culture of Nodes and Internodes
Mohammed L’bachir El Kbiach1, Brahim El Bouzdoudi1*, Rabah Saïdi2, Zineb Nejjar El Ansari1, Safaa Rahmouni1, Ahmed Lamarti1
1Laboratory of Plant Biotechnology, Biology Department, Faculty of Sciences, Abdelmalek Essaadi University, Tetouan, Morocco
2Department of Matter and Life Sciences, High Normal School, Martil, Morocco

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: June 9, 2017; Accepted: July 3, 2017; Published: July 6, 2017
ABSTRACT
The present study is about in vitro culture of cork oak, through callogenesis from nodes and internodes, withdrawn from seedlings of three months. These latter were obtained after acorns germination on peat. Nodes showed a high capacity for callogenesis and the best rate was obtained on a medium containing Woody Plant Medium (WPM) macronutrients, Murashige and Skoog (MS) micronutrients and vitamins, 4.5 µM of 6-Benzylaminopurine (BAP), 7 µM of 2-naphthalineacetic acid (NAA) and 30 g/L of Sucrose. Calluses are transplanted onto the same mineral solution, with or without growth regulators, and in the 3rd transplanting; small white embryos appear on the surface of calluses.
Keywords:
Cork oak, Quercus suber L., Nodes, Internodes, Callogenesis

1. Introduction
Moroccan forests have many advantages related to their impact on the socio-economic balance of the country, their contribution to the development of the rural areas and their high biological diversity. They play a major role in meeting the country’s wood needs, protecting soil against erosion, preserving water, providing population with various renewable natural resources and improving environment conditions. In addition, these forests constitute an irreplaceable reservoir of genes of various plant species of great utility for society. Morocco has a forest area of nearly 9 million hectares, which represents around 12.7% of the total area of the country. The area of the natural forest amounts to nearly 5.8 million hectares, while reforestation covers 586,000 ha. These forests are also characterized by an important diversity, favored by the varied conditions of relief, geology and climate of the country. Thus, there are no less than 270 woody plants, including 68 forest species, 16 main and 52 subordinate. Moreover, 19 regions of forests have been delimited [1] [2] [3] [4] .
Actually, a considerable success has been achieved in the production of plants from calli cultures, for some Angiosperm species [5] . Such a culture can be initiated from any explants capable of dividing on an appropriate nutrient medium and under aseptic conditions [6] [7] [8] [9] .
For the cork oak (Quercus suber), very few studies have been established; the first experiments date backs to Jacquiot [10] who succeeded in obtaining calluses by in vitro culture of the cambial tissue, but without regenerating complete plant organs.
Calluses may be an intermediate stage in the formation of somatic embryos of Quercus suber. Thus, El Maataoui and Espagnac [11] , Feraud-Keller et al. [12] and El Maataoui et al. [13] were able to obtain somatic embryos from the calluses induced on internodes of cork oak. Thereby, we have oriented our research on the callogenesis of the nodes of Quercus suber.
Actually, the proliferation of explants nodes depends essentially on the nutritive factors and growth regulators present in the culture medium. In addition, positions of the fragments and exposure to light have an influence on callogenesis during culture. Moreover, successive subcultures of the calluses were carried out in order to obtain somatic embryos.
2. Material and Methods
2.1. Plant Material
Calluses are obtained from explants cultured on a suitable medium. For this purpose, we tried to optimize callogenesis of cork oak by using as starting material nodes and internodes (1 to 1.5 cm in length) taken from 3-month-old seedlings obtained from acorn germination on the peat.
2.2. Sterilization of Nodes and Internodes
The seedlings are washed with running water to remove any trace of pesticides. After several attempts to sterilize the nodes and internodes of the cork oak, we adopted the sterilization protocol with a 7% (w/v) filtered solution of calcium hypochlorite (Ca(OCl)2) of about 120˚, containing few drops of Tween 80 for 20 min, then with a 0.1% (w/v) solution of mercuric chloride (HgCl2) for 2 min. Finally, HgCl2 is removed by 4 successive rinses of 5, 5, 10 and 15 minutes in sterile distilled water.
2.3. Calluses Induction
2.3.1. From Nodes
1) Effect of Mineral Solution
In order to stimulate callus formation from the nodes, these latter were cultured on several solutions of macronutrients, different in composition and especially in the concentration of these nutrients. The macronutrients tested are those of Woody Plant Medium (WPM) [14] , Murashige and Skoog (MS) [15] , and Schenk and Hildebrandt (SH) [16] , to which we added MS micronutrients and vitamins. These mediums are supplemented with myo-inositol and sucrose (100 mg/L and 30 g/L, respectively). BAP is used at 4.5 μM and NAA at 7 μM.
2) Modifications Made in WPM Medium
These changes concern:
- Sucrose, replaced by as much (30 g/L) glucose;
- The addition to the base medium of organic nitrogen through glutamine, at different concentrations (0.5 and 1 g/L);
- The amine complex, casein hydrolyzate, tested at 0.5 and 1 g/L.
The effect of darkness was also studied. Other changes were also taken into account. Thus, the nodes were cultured horizontally and vertically on the base medium to see the influence of explant’s position on callogenesis.
3) Growth Regulators Effect
In order to develop the appropriate medium for the formation of calluses from the nodes, it has proved necessary to test several hormonal combinations. Thus, different cytokinins (6-Benzylaminopurine; BAP, Kinetin and Zeatin), Adenine and compounds derived from phenylurea (Diphenylurea; DPU and Thidiazuron; TDZ) were used alone or in combination with auxins (2-naphtha- lineacetic acid; NAA, Indole-3-butyric acid; IBA and Indole-3-acetic acid; IAA). The Gibberellic acid (GA3) combined with BAP was also tested.
2.3.2. From Internodes
Internodes of 1 to 1.5 cm in length, excised from seedlings of 3 months and sterilized by the same protocol used for the nodes, were cultured horizontally and vertically, on the same base medium used for the nodes.
2.4. Calluses Transplanting
Successive subcultures (every two weeks) were done on the base medium to monitor calluses evolution over time. Other subcultures were also carried out on the base medium:
- Devoid of any growth substance and exposed to light (16-hour photoperiod at 60 μmol∙m−2∙s−1 provided by fluorescent lights, cold and white);
- Free from growth regulators and placed in the dark;
- Added with 2,4-Dichlorophenoxyacetic acid (2,4-D) at different concentrations (between 2.3 and 22.6 μM) and exposed to light (16-hour photoperiod at 60 μmol∙m−2∙s−1 provided by fluorescent lights, cold and white);
- Supplemented with BAP (2.2; 4.5; 9; 22.5 and 45 μM) and exposed to light (16-hour photoperiod at 60 μmol∙m−2∙s−1 provided by fluorescent lights, cold and white).
2.5. Measurement of Calluses Growth
The growth of calluses neoformed from the nodes was evaluated primarily based on the weight of fresh material.
2.5.1. Weighing Method
The purpose of this operation is to determine the weights of fresh material of calluses during their growth. The method includes some measurements:
- A set of calluses is taken out of flasks, carefully separated from the agar, wiped lightly and then placed on a sheet of aluminum previously weighed. The whole is weighed (weight of fresh starting material) and calluses are transplanted onto a new medium;
- Every two weeks, calluses are again weighed to establish the evolution of their weight of fresh matter.
2.5.2. Growth Index
Growth index (1) is the ratio:
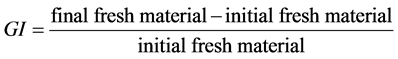 (1)
(1)
This growth index corresponds to Klein’s “growth increment” [17] , with the difference that he estimated it as a percentage.
2.6. Statistical Analysis
For all these tests, 30 explants were used for each experiment. The experiments were repeated 3 times. One and two-way analysis of variance (ANOVA) was carried out to determine differences between each treatments. Multiple comparisons were made using Tukey post-hoc test (p ≤ 0.05).
3. Results
3.1. Calluses Induction
3.1.1. Macronutrients Effect
Fragments of nodes that length is 1 to 1.5 cm, taken from cork oak seedlings were cultured vertically, half-embedded on agar media. We compared the action of three mineral solutions commonly used in the laboratory for plant tissues culture. These are the macronutrients of MS, SH and WPM supplemented with 7 μM NAA combined with 4.5 μM BAP. To these media, 30 g/L of sucrose is added as an energy source. The results are shown in Table 1.
After 10 days of culture, blisters packed against each other appear around the petiole covering the axillary bud. These blisters then extend to the apical and lateral surfaces of the explants, which are situated outside the medium. They evolve to produce a soft whitish callus that gradually covers the entire surface of the explants. After 3 weeks, the callus is whitish, with little chlorophyll, compact and friable.
Callogenesis depends on the mineral solution used. Thus, we recorded a maximum number of callogenous explants on the WPM macronutrients solution (90%). Calluses obtained are well developed, with a diameter of 0.7 to 1.1 cm. On MS and SH macronutrients, the percentage of callogenesis is low (22.2
Table 1. Effect of mineral solution on the formation of calluses, obtained from nodes of cork oak seedlings, after one month of growth in the presence of BAP at 4.5 µM and NAA at 7 µM.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
and 26.7% respectively) and the callus is poorly developed (maximum diameter 0.5 cm). We therefore retained the WPM macronutrients for subsequent experiments.
3.1.2. Glucose Effect
Substitution of sucrose with the same amount of glucose (30 g/L) decreases the percentage of callogenesis (53.3%). The neoformed callus is always whitish, compact, friable and moderately developed (diameter is between 0.4 and 0.7 cm) (Table 2).
3.1.3. Glutamine Effect
Glutamine at different concentrations (0 - 0.5 and 1 g/L) was tested on callus formation (Table 3).
The increase in glutamine content reduces considerably nodes callogenesis (16.7% to 1 g/L). The toxic effects of high levels of glutamine are manifested by total tissue necrosis, accompanied by growth stopping after 30 days of culture. The formed calluses are compact, friable and poorly developed.
3.1.4. Effect of Casein Hydrolyzate
The influence of casein hydrolyzate content on the callogenesis of the nodes is reported in Table 4.
The presence of casein hydrolyzate in the culture medium is unfavorable to callogenesis (13.3% at 0.5 g/L and 10% at 1 g/L). Neoformed calluses in the presence of casein hydrolyzate are always compact, friable and very poorly developed (0.2 to 0.5 cm in diameter). From the second week of culture, a necrotic aspect of callus appears and is accentuated after 20 days of culture. Thus, casein hydrolyzate has a toxic effect on the callogenesis of cork oak nodes.
3.1.5. Darkness Effect
In the same medium as previously, nodes exposed to light (16-hour photoperiod) showed high callogenesis (90%) compared to those exposed to darkness (36.7%) (Table 5), in addition, calluses formed in the dark are generally compact and friable, but not very developed (0.2 - 0.3 cm in diameter) compared to those
Table 2. Effect of 30 g/L of glucose on the formation of callus from cork oak seedlings nodes, after one month of growth, on WPM medium added with 4.5 µM of BAP and 7 µM of NAA.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
Table 3. Effect of glutamine on callus formation from cork oak seedlings nodes, after one month of growth on WPM medium, added with 4.5 µM of BAP and 7 µM of NAA.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
Table 4. Effect of casein hydrolyzate on callus formation from cork oak seedlings nodes, after one month of growth in WPM medium, added with 4.5 µM of BAP and 7 µM of NAA.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
Table 5. Effect of darkness on callus formation from cork oak seedlings nodes, after one month of growth on WPM medium, added with 4.5 µM of BAP and 7 µM of NAA.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
exposed to a 16-hour photoperiod.
After two weeks, calluses growing in the dark brown and their growth stops. Their weight of fresh material is low (0.12 g) compared to that obtained with light (0.31 g).
3.1.6. Effect of Growth Regulators
1) Cytokinins
A series of preliminary experiments were undertaken to establish the in vitro culture of cork oak explants, in the presence of various doses of cytokinins (BAP, Kinetin and Zeatin), Adenine and compounds with the same activity of cytokinins (DPU and TDZ). The essential purpose of these experiments was to observe the effect of every growth regulator on the formation of a primal callus. The results are reported in Table 6.
The tissue responses to BAP, Kinetin and DPU were identical. The presence of these substances does not promote callogenesis; the percentage of explants forming callus is zero.
Zeatin induces callus formation regardless of the concentration used. The maximum of callogenesis is observed at 2 μM (74%) and the minimum at 4.5 µM (41%). The callus is generally white, compact, hard and undeveloped.
After a week of growth in the presence of Adenine, blisters packed against each other appear around the petiole surrounding the axillary bud. Then, they evolve to produce a chlorophyll callus. Callogenesis is significant at 2 μM (80%) and tends to decrease with concentration. The calluses are not very developed, green, compact and hard.
In the presence of thidiazuron, the percentage of callogenesis is important and increases with the concentration (maximum of 91% at 7 μM). The callus is green, compact and not very developed.
2) BAP Combined to Auxins and GA3
Following studies carried out by Das et al. [18] , Skoog and Miller [19] , Letham [20] and Heller [21] , we still admit that cytokinins act synergistically with auxins to induce cell division and allow the proliferation of plant tissues cultured in vitro. The behavior of these tissues varies according to the combinations of growth regulators used.
For this reason, we have combined BAP (4.5 μM) with different doses of auxins (NAA, IBA or IAA) or GA3. Callogenesis induced by the nodes was evaluated after 30 days of culture (Table 7).
The NAA clearly favors callogenesis, with a maximum of 93% at 7 μM. Calluses are vigorous, white, compact and friable. Diameter can reach 1.1 cm (Figure 1(a) and Figure 1(b)).
IBA induces callogenesis, with a maximum of 78% at 5 μM. Calluses are white, compact and friable (Figure 1(c)). Actually, the addition of IBA didn’t really exalt proliferation since calluses diameters remain low compared to those obtained in the presence of NAA.
Important percentages of callogenesis were recorded for all IAA concentrations (Figure 1(d)). The neoformed calluses are of medium size (diameter from
Table 6. Effect of three cytokinins, adenine and two compounds derived from phenylurea (DPU and TDZ), on callus formation from cork oak nodes, after 4 weeks of growth in WPM medium.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
Table 7. Effect of BAP (4.5 µM), combined with 3 auxins (NAA, IBA or IAA) and with GA3 in different concentrations, on callus formation from cork oak nodes, after 4 weeks of growth on WPM medium.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
0.4 to 0.7 cm), white, compact and friable.
The cytokinin incorporated in WPM medium, alone or in combination with GA3, doesn’t induce cork oak nodes proliferation, which has prompted us to stop experiments with gibberellic acid.
From these results, it can be concluded that BAP alone has a clearly inhibiting effect on the induction of callus. However, associated with auxins, cytokinin further enhances the stimulatory effect of auxins on callogenesis.
3.1.7. Effect of the Nature of Explant
Inter-nodes and nodes fragments were cultured horizontally and vertically on WPM medium supplemented with 4.5 μM of BAP combined with 7 μM of NAA. The results are summarized in Table 8.
Figure 1. Callogenesis of nodes obtained from young seedlings of the cork oak (a); (b) Callus after 30 days of culture of the nodes on WPM medium supplemented with 4.5 μM of the BAP and 7 μM of NAA; (c) Callus after 30 days of culture of the nodes on WPM medium supplemented with 4.5 μM of BAP and 5 μM of IBA. It develops around the axillary bud (arrow); (d) Callus after 30 days of culture of the nodes on WPM medium supplemented with 4.5 μM of BAP and 2.5 μM of IAA; (e) Callus after 30 days of horizontal culture of the internodes on WPM medium supplemented with 4.5 μM of BAP and 7 μM of NAA; (f); (g) Callus obtained from the nodes, after 2 successive subcultures on WPM medium supplemented with 4.5 μM of BAP and 7 μM of NAA; (h) Callus obtained from the nodes, after 3 successive subcultures on WPM medium supplemented with 4.5 μM of BAP.
The horizontal culture of the nodes decreases the percentage of callogenesis from 90% (vertical culture) to 76.6%. Similarly, calluses are less developed in horizontal culture and their diameter varies between 0.4 and 0.8 cm. These calluses are always white, compact and friable, they develop only on the upper surface of the explant (which is not in contact with the medium). As a result, we retained the vertical culture for subsequent experiments.
For the internodes, a slight swelling is observed from the second week of cul-
Table 8. Effect of the nature of the explant and position (horizontal H and vertical V) on callus formation for cork oak, after one month of growth on WPM medium supplemented with 4.5 µM of BAP and 7 µM of NAA.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
ture, affecting the end situated outside the medium. After 4 weeks, small pustules appear at the base of the explants and then fuse to form an hemispherical callus, hilly and friable; This latter progressively covers the entire surface of the explants.
After 6 weeks of culture, callus is chlorophyllian and preserves its appearance and consistency.
After passage on a new medium containing 4.5 μM of BAP combined with 7 μM of NAA, some cells undergo a loss of chlorophyll. The calluses brown and their growth are totally inhibited after 8 weeks of culture.
Nevertheless, we observed that the primary calluses of the internodes can give rise, after three monthly subcultures to a medium without growth regulators, to small white somatic embryos developing on the surface of the calluses.
The percentage of callogenesis is important in both horizontal and vertical culture (80% and 77.4%, respectively) (Figure 1(e)). Generally, calluses have a diameter ranging from 0.5 to 0.7 cm (Table 8).
Furthermore, although the percentage of callogenesis in the internodes is large, the proliferation remains limited since the average weight of fresh matter expressed in g per fragment is low (0.06 g in horizontal culture and 0.08 g in vertical culture) compared to that obtained in the nodes (0.13 g in horizontal culture and 0.30 g in vertical culture).
Similar results were obtained for horizontal or vertical culture of nodes and internodes on medium of MS medium supplemented with 20 g/L of glucose, 10 μM of IBA and 8.88 μM of BAP, which turns out to be toxic to callogenesis (Table 9). The percentage of callogenesis is low or zero.
We retained the combination of 4.5 μM of BAP and 7 μM of NAA, on which we replanted calluses (Figure 1(f) and Figure 1(g)).
Growth follow-up is shown in Figure 2. Calluses growth is slow and stops after 13 days. Beyond that, it is useless to continue culturing.
Figure 2. Time-dependent variation in growth index (GI) of fresh and dry matter of cork oak callus after transplanting on WPM medium supplemented with 4.5 μM of BAP and 7 μM of NAA.
Table 9. Effect of MS medium added with 2% of glucose, 2 mg/L of IBA and 2 mg/L of BAP, on the formation of callus from cork oak seedlings nodes and internodes, after one month of horizontal culture.
Values in the same column that are followed by the common letter do not differ significantly from each other according to the Tukey post-hoc multiple range test (p ≤ 0.05).
3.2. Calluses Transplanting
Calluses transplanting was performed in different concentrations of BAP (2.2; 4.5; 9; 22.5 and 45 μM) or 2,4-D (2.3; 4.5; 9 and 22.6 μM).
After 6 weeks of culture in the presence of BAP, results of the preliminary tests relating to the induction of caulogenesis highlight the total absence of budding from calluses nodes of cork oak (Figure 1(h)). Chlorophyll appears on calluses after 3 weeks of culture. These proliferating calluses are mainly embryogenic calluses. Thus, we recorded the neoformation of somatic embryos of reduced size on the surface of calluses. These somatic embryos are white and easily detach from the callus of origin.
Gradually as the BAP concentration increases, the average weight of the fresh material expressed in g per explant decreases (Figure 3).
Similarly, in the presence of 2,4-D in the culture medium, calluses are always embryogenic and of white color. Necrosis appears as early as the fourth week of culture. The average weight of fresh material expressed in g per explant is relatively higher than that obtained in the presence of BAP and increases with increasing concentrations of the auxin (Figure 4).
Transplanted to light on a medium without growth regulators, calluses retain their white and embryogenic appearance. However, at the next subculture on growth-free medium and in the dark, the average weight of fresh material decreases from 1.43 g to 1.14 g and the callus browns immediately after the second
Figure 3. Fresh material weight change according to BAP concentration, for cork oak callus.
Figure 4. Fresh material weight change according to 2,4-D concentration, for cork oak callus.
week of culture.
A third transplant on a medium containing 4.5 μM of BAP and 7 μM of NAA and in light doesn’t give satisfactory results. 15 days after transplanting of the tertiary calluses, their growth stops and there is no need to extend cultures (Figure 5).
4. Discussion
In our preliminary experiments on the induction of callogenesis from the nodes, we tested three solutions of macronutrients, different in composition and in concentrations, namely MS, SH and WPM. After four weeks of culture, WPM solution proved to be the most suitable. The high levels of nitrogen ammonia exert an inhibitory action on the formation of calluses, what coincides with the results recorded by Flick et al. [22] and Aboel-Nil [23] , for whom high concentrations of 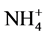 reduce cell proliferation in some cultures.
reduce cell proliferation in some cultures.
The substitution of sucrose by glucose was unfavorable to nodes callogenesis, whereas the latter sugar was successfully used for the induction of calluses on internodes of cork oak [11] [12] [13] .
The addition of glutamine or casein hydrolyzate weakens callogenesis. How-
Figure 5. Time-dependent variation in growth index (GI) of fresh and dry matter of cork oak callus after the transplanting on WPM medium supplemented with 4.5 μM of BAP and 7 μM of NAA.
ever, two weeks after they were cultured in the presence of 1 g/L of casein hydrolyzate, all inter-node fragments began to emit more or less voluminous whitish calluses. They first appear at both ends of the fragments before emerging from the lenticels and covering all the explants. Detached from the internodes that spawned them, and regularly transplanted on a fresh medium, these calluses continue to proliferate, take a granular appearance with chlorophyll [11] [12] .
This type of response has also been obtained in cork oak by culture of cambial tissues [10] [24] and in green oak (Quercus ilex L.) from foliar tissues [25] .
Similarly, from leaves of 6 mm diameter excised from young seedlings of Quercus rubra (6 to 8 weeks after germination), a callogenesis was obtained on mineral medium Gamborg (B5) [26] supplemented with 0.5 g/L of casein hydrolyzate, 30 g/L of sucrose, 9 μM of 2,4-D and 0.9 μM of BAP or 26.7 μM of NAA and 4.5 μM of BAP [27] . Pinto et al. [28] observed callogenesis in 2- month-old leaves from Quercus suber cuttings grown in the dark on MS medium, supplemented with 4.5 μM of 2,4-D and 0.04 μM of BAP or 9 μM of 2,4-D and 2.0 μM of BAP. Loureiro et al. [29] obtained important embryogenic callus formations on leaves from Quercus suber cuttings. The medium used is that of MS in the presence of 2,4-D (4.5 μM) and zeatin (9 μM). In our case, the combination zeatin/2,4-D doesn’t favor callogenesis.
The induction of callogenesis is better when the explants are exposed to light. In the dark, the majority of newly formed calluses turn brown, their growth stops and they become necrotic. These observations are similar to those of El Maataoui et al. [13] .
However, calluses were obtained from Quercus glauca leaves segments grown in the dark on half-diluted MS medium and supplemented with 10.7 μM of NAA and 0.44 μM of BAP. These calluses, detached from the segments that spawned them, were able to be transplanted monthly on the same medium, in the dark and for 6 months [30] . Similarly, Krajci and Gross [31] obtained calluses after culturing Quercus robur stem segments excised from 1 to 3 month old seedlings on a solid medium of Linsmaier and Skoog (LS) [32] in the dark and at 25 ˚C. These calluses were then studied for their tannins content.
The culture of the nodes and inter-nodes on MS medium supplemented with 20 g/L of glucose, 10 μM of IBA and 8.88 μM of BAP proves to be toxic under our experimental conditions. However, satisfactory results have been recorded by our predecessors for Quercus suber internodes culture on the same nutrient medium [11] [12] [13] .
BAP alone at the concentrations used doesn’t promote the formation of calluses. However, it allowed significant callogenesis rates from Quercus robur nodes, especially at 1 μM (91.6%), with compact and nodular calluses [33] .
It should be noted that this is the first time that we obtain calluses from Quercus suber nodes in the presence of thidiazuron [34] .
Among the auxins tested in combination with BAP, NAA gives the best percentages of callogenesis (87% and 93% at 5 and 7 μM). NAA/BAP combination is commonly used for callus induction. Thus, in Quercus rubra, Seckinger et al. [35] [36] obtained a dense, yellow-white, nodular callus developing at the periphery of the stem segments grown for one month on a modified MS medium containing these two growth regulators.
According to Cuenca et al. [37] , Quercus robur internodes were grown in the dark on MS medium supplemented with casein hydrolyzate (0.5 g/L), sucrose (30 g/L), BAP (2.2; 4.5 and 8.9 μM) and NAA or IBA (1; 2 and 4 mg/L). After two weeks of culture, 90% to 100% of the explants have become callogenous, especially in the presence of NAA. Chlorophyll appears on the white calluses after transfer to the light.
Successive subcultures (three subcultures) on the same base medium without growth regulators or containing different concentrations of BAP or 2,4-D, made it possible to obtain, on the surface of the calluses, structures reminiscent of somatic embryos. These latter are small in size and have a white appearance.
In our experiments, obtaining somatic embryos indirectly (a phenomenon necessitating establishing callus beforehand) is somewhat random (7% of cases). Moreover, El Maataoui and Espagnac [11] noted in a small number of cases (about 3%), after 5 to 7 weeks of culture without transplanting, the appearance on calluses surface, of small blisters peeling off with ease. Most of them evolve rapidly into small nodules, first spherical and then taking an elongated form and differentiating, on one end, a root apex and, on the opposite end, two elements reminiscent of the cotyledons of a young seedling.
Similarly, in the Green Oak, the first calluses appear after 4 days. After 7 months of culture without any transplanting, individual small spherical protuberances appear on the surface of the calluses. These primary nodules are transplanted separately. Exposed to light, they proliferate in calluses with chlorophyll and without any organogenesis. Some (3%) of those cultured in the dark produce new protuberances or secondary nodules, that transplanted in both light and dark produce somatic embryos [12] .
Previously, Seckinger et al. [35] observed small nodules on the periphery of calluses produced by internodes of young Quercus rubra plants, which they described as organoids. But these formations, entirely parenchymatous, had no meristematic zone and showed no further development.
In 1994, Rancillac et al. [27] observed the formation of somatic embryos after 6 to 8 weeks on calluses from leaf discs, from 3 to 6 weeks old and 10 years old Quercus rubra. The embryos have cotyledons and a functional root pole; contrariwise, the caulinary pole is devoid of meristem.
Regular transplanting of calluses has an inhibiting role in the establishment of somatic embryogenesis. We can think that the fragmentation practiced at the time of the transplantation restarts the proliferation and thus prevents the callus from morphogenesis [12] .
5. Conclusions
Although some studies have shown the possibility of obtaining calluses from various explants of cork oak (inter-nodes, leaves, zygotic embryos, etc.), this is the first time, at least to our knowledge, that true cell proliferations are obtained from the nodes.
We have investigated the conditions for node callogenesis and it has been found that the WPM macronutrients are the most suitable. The choice of the nature of the carbon source is a determining factor in the formation of calluses. In fact, the substitution of sucrose by glucose decreases the rate of callogenesis.
We have also observed that glutamine and casein hydrolyzate have markedly inhibitory effects on the formation of calluses from the nodes of Quercus suber grown in vitro. Similarly, culturing nodes in dark was unfavorable both in terms of callogenesis rate and the appearance and size of neoformed calluses. This dark is also inappropriate to the transplants of calluses.
In addition, we have shown by studying the effect of the positioning of the explants that the vertical culture of the nodes gives the best percentage of callogenesis. Similarly, the choice of the nature of the explant is an important factor: cork oak nodes are more able to form well-developed calluses than inter-nodes.
We have also shown that significant cell proliferation in cork oak requires the use of powerful synthetic auxins and that BAP seems often act in synergy with NAA to stimulate callogenesis. Thus, the NAA/BAP selected during callogenesis phase is 7 μM NAA and 4.5 μM BAP.
Successive subcultures of the calluses favor the formation, on their surface, of small, white somatic embryos. These formations appear both in the presence and absence of growth substances.
We also induced callogenesis on the embryonic axis. Thus, adding NAA to the culture medium containing BAP (0.44 μM) blocks the growth of stem and roots, promotes the formation of a white callus and sometimes the formation of several lateral roots around the main root.
Cite this paper
El Kbiach, M.L., El Bouzdoudi, B., Saïdi, R., Nejjar El Ansari, Z., Rahmouni, S. and Lamarti, A. (2017) Callo- genesis of Cork Oak (Quercus suber L.) through In Vitro Culture of Nodes and In- ternodes. American Journal of Plant Scien- ces, 8, 1801-1819. https://doi.org/10.4236/ajps.2017.88123
References
- 1. Ajbilou, R., Maranón, T. and Arroyo, J. (2006) Ecological and Biogeographical Analyses of Mediterranean Forests of Northern Morocco. Acta Oecologica, 29, 104-113.
https://doi.org/10.1016/j.actao.2005.08.006 - 2. Benzyane, M. (1997) Improvement Strategy of Forest Tree Species in Morocco: The Case of Cork Oak. Quercus Suber Network. Report of the Third and Fourth Meetings, 9-12 June 1996 (Italy), 20-22 February 1997 (Spain), 60-67.
- 3. Benzyane, M. (1998) The Moroccan Forest, Economic and Social Product to Be Developed. In: Proceedings of the Mediterranean Seminar on the Vegetation of Cork Oak Forests. Tabarka, 22-24 October 1996, Annals of INGREF, Special Issue, 12-21.
- 4. Boudy, P. (1950) North African Forest Economy. Monograph and Treatment of Tree Species. Larose, Paris, 525.
- 5. Bajaj, Y.P.S. (1986) Biotechnology of Tree Improvement for Rapid Propagation and Biomass Energy Production. In: Biotechnology in Agriculture and Forestry, Chapter Trees I, Vol 1, Springer-Verlag, Berlin, 1-23.
https://doi.org/10.1007/978-3-642-70576-2_1 - 6. Vasil, I. and Hildebrandt, A. (1966) Variations of Morphogenetic Behaviour in Plants Tissue Cultures. II. Petroselinum hortense. American Journal of Botany, 53, 869-874.
https://doi.org/10.2307/2439808 - 7. Banerjee, S. and Gupta, S. (1975) Morphogenesis in Tissue Cultures of Different Organes of Nigella sativa. Physiologia Plantarum, 33, 185-187.
https://doi.org/10.1111/j.1399-3054.1975.tb03790.x - 8. Hunault, G. (1979) Research on Behavior of Organ and Tissue Fragments of In Vitro Cultivated Monocotyledons. III. Study of the Case of Several Liliaceae. Revue de cytologie et de biologie végétales, 2, 103-154.
- 9. Novak, F.J., Opatrny, Z., Rovenska, B. and Nesticky, M. (1979) Studies on the Morphogenetic Response of Maize Tissue Cultures of Different Origin. Biologia Plantarum, 21, 418-426.
https://doi.org/10.1007/BF02889482 - 10. Jacquiot, C. (1952) On the Phenomena of Histogenesis Observed in In Vitro Cultures of Cambia Tissue of Oaks (Quercus sessiliflora Sm., Quercus pedunculata Ehr., Q. suber L.). Comptes rendus hebdomadaires des séances de l’Académie des sciences, 234, 1468-1470.
- 11. El Maataoui, M. and Espagnac, H. (1987) Neoformation of Somatic Embryonic Type Structures on Cork Oak Tissue Cultures (Quercus suber L.). Comptes Rendus de l’Académie des Sciences. Série 3, 304, 83-88.
- 12. Feraud-Keller, C., El Maataoui, M., Gouin, O. and Espagnac, H. (1989) Somatic Embryogenesis in three Species of Mediterranean Oaks. Annals of Forest Science, 46, 130-132.
- 13. El Maataoui, M., Espagnac, H. and Michaux-Ferriere, N. (1990) Histology of Callogenesis and Somatic Embryogenesis Induced in Stem Fragments of Cork Oak (Quercus suber) Cultured In Vitro. Annals of Botany, 66, 183-190.
https://doi.org/10.1093/oxfordjournals.aob.a088014 - 14. McCown, B.H. and Lloyd, G. (1981) Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture of Woody Plant Species. HortScience, 16, 453-453.
- 15. Murashige, T. and Skoog, F. (1962) A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Culture. Physiologia Plantarum, 15, 473-497.
https://doi.org/10.1111/j.1399-3054.1962.tb08052.x - 16. Schenk, R.V. and Hildebrandt, A.C. (1972) Medium and Techniques for Induction and Growth of Monocotyledonous and Dicotyledonous Plant Cell Cultures. Canadian Journal of Botany, 50, 199-204.
https://doi.org/10.1139/b72-026 - 17. Klein, R.M. (1963) Interaction of Ultraviolet and Visible Radiation on the Growth of Cell Aggregates of Ginkgo Pollen Tissue. Physiologia Plantarum, 16, 73-81.
https://doi.org/10.1111/j.1399-3054.1963.tb08291.x - 18. Das, N.K., Patau, K. and Skoog, F. (1956) Initiation of Mitosis and Cell Division by Kinetin and Indolacetic Acid in Excised Tobacco Pith Tissue. Physiologia Plantarum, 9, 640-651.
https://doi.org/10.1111/j.1399-3054.1956.tb07826.x - 19. Skoog, F. and Miller, C.O. (1957) Chemical Regulation of Growth and Organ Formation in Plant Tissues Cultured In Vitro. Symposia of the Society for Experimental Biology, 11, 118-131.
- 20. Letham, D.S. (1969) Cytokinins and Their Relation to Other Phytohormones. Bio-Science, 19, 309-316.
https://doi.org/10.2307/1294513 - 21. Heller, R. (1978) Abstract of Vegetal Physiology. Volume II: Development. Masson, Paris.
- 22. Flick, C.E., Evans, D.A. and Sharp, W.R. (1983) Organogenesis. In: Sharp, W.R., Evans, D.A., Ammirato, P.V. and Yamada, Y., Eds., Handbook of Plant Cell Culture, Vol. 1, Macmillan Publishing Company, New York, 13-81.
- 23. Aboel-Nil, M.M. (1987) Tissue Culture of Douglas-Fir and Western North American Conifers. In: Bonga, J.M. and Durzan, D.J., Eds., Cell and Tissue Culture in Forestry, Vol. 3, Martinus Nijhoff Publishers, Dordrecht, 80-100.
https://doi.org/10.1007/978-94-017-0992-7_6 - 24. Jacquiot, C. (1956) On the Requirements in Auxines and the External Morphological Characters of the Cultures of Cambial Tissue of some Tree Species. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences, 243, 510-512.
- 25. Feraud, C. (1985) Some Aspects of Green Oak Growth and Development (Quercus ilex L.) and First In Vitro Culture Tests. Thesis of 3rd Cycle, Faculty of Sciences and Techniques of Saint-Jérome, Aix-Marseille III.
- 26. Gamborg, O.L. (1966) Aromatic Metabolism in Plants. II. Enzymes of the Shikimate Pathway in Suspension Cultures of Plant Cells. Canadian Journal of Biochemistry, 44, 791-799.
https://doi.org/10.1139/o66-097 - 27. Rancillac, M., El-Hamri, I. and Klinguer, A. (1994) Expression of Somatic Embryogenesis from Leaf Discs in a Forest Tree, the American Red Oak. Plant Science, 78.
- 28. Pinto, G., Valentim, H., Costa, A., Castro, S. and Santos, C. (2002) Somatic Embryogenesis in Leaf Callus from a Mature Quercus suber L. Tree. In Vitro Cellular and Developmental Biology Plant, 38, 569-572.
https://doi.org/10.1079/IVP2002352 - 29. Loureiro, J., Pinto, G., Lopes, T., Dolezel, J. and Santos, C. (2005) Assessment of Ploidy Stability of the Somatic Embryogenesis Process in Quercus suber L. Using Flow Cytometry. Planta, 221, 815-822.
https://doi.org/10.1007/s00425-005-1492-x - 30. Nishikawa, K., Tanaka, N., Nakanishi, F., Shimomura, K. and Ishimaru, K. (1997) Tannins and Related Phenolics in Callus and Root Cultures of Quercus glauca. Plant Biotechnology, 14, 123-125.
https://doi.org/10.5511/plantbiotechnology.14.123 - 31. Krajci, I. and Gross, G.G. (1987) Formation of Gallotannins in Callus Cultures from Oak (Quercus robur). Phytochemistry, 26, 141-143.
- 32. Linsmaier, E.M. and Skoog, F. (1965) Organic Growth Factor Requirements of Tobacco Tissue Cultures. Physiologia Plantarum, 18, 100-127.
https://doi.org/10.1111/j.1399-3054.1965.tb06874.x - 33. Pevalek-Kozlina, B. and Jelaska, S. (1995) The Regeneration Ability in Common Oak (Quercus robur L.) Callus Cultures. Acta Pharmaceutica, 45, 165-398.
- 34. El Kbiach, M.L., Lamarti, A. and Badoc, A. (2001) In Vitro Culture of Axillary Buds of Cork Oak (Quercus suber L.). I. Influence of Cytokinins on the Organogenesis and Callogenesis of Seed Nodes. Bulletin de la Société de Pharmacie de Bordeaux, 140, 67-82.
- 35. Seckinger, G.R., McCown, B.H. and Struckmeyer, B.E. (1979) Production of Anomalous Structures in Quercus rubra L. Callus Cultures. American Journal of Botany, 66, 993-996.
https://doi.org/10.2307/2442244 - 36. Seckinger, G.R., McCown, B.H., Struckmeyer, B.E. and Durbin, R.D. (1978) Production and Rapid Multiplication of Organoids from Callus Cultures of Quercus rubra L. (Red Oak). HortScience, 13, 355-355.
- 37. Cuenca, B., San-Jose, M.C., Martinez, M.T., Ballester, A. and Vieitez, A.M. (1999) Somatic Embryogenesis from Stem and Leaf Explants of Quercus robur L. Plant Cell Reports, 18, 538-543.
https://doi.org/10.1007/s002990050618







