Open Journal of Respiratory Diseases
Vol.07 No.02(2017), Article ID:76608,19 pages
10.4236/ojrd.2017.72009
Efficacy of a Topical Aromatic Rub (Vicks VapoRub®) on Effects on Self-Reported and Actigraphically Assessed Aspects of Sleep in Common Cold Patients
Nayantara Santhi1, David Ramsey2, Gill Phillipson3, David Hull3*, Victoria L. Revell4, Derk-Jan Dijk1
1Surrey Sleep Research Centre, School of Biosciences and Medicine, Faculty of Health and Medical Sciences, University of Surrey, Surrey, UK
2The Procter & Gamble Company, Mason Business Center, Mason Montgomery Road, Mason, OH, USA
3Procter & Gamble, Greater London Innovation Centre, Whitehall Lane, Egham, Surrey, UK
4Surrey-Clinical Research Centre, Department of Clinical and Experimental Medicine, School of Biosciences and Medicine, Faculty of Health and Medical Sciences, University of Surrey, Surrey, UK

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 25, 2017; Accepted: May 24, 2017; Published: May 27, 2017
ABSTRACT
Common cold sufferers frequently report sleep disruption during the symptomatic period of infections. We examined the effects of treatment with a topical aromatic pharmaceutical ointment (Vicks VapoRub®), on associated sleep disturbances. The effects of Vicks VapoRub® versus placebo (petrolatum ointment) on subjective and objective measured sleep parameters were assessed in an exploratory study of 100 common cold patients, in a randomized, single blind, controlled, two-arm, parallel design study. The primary efficacy variable was subjective sleep quality measured with the SQSQ (Subjective Quality of Sleep Questionnaire). Additional measures included, ease of falling asleep and depth of sleep (measured with a post-sleep Visual Analog Scale), total sleep time, sleep onset latency, activity score, percentage of sleep, sleep efficiency (measured with actigraphy and SQSQ) and sleep quality index measured with a modified Karolinska Sleep Diary (KSD). The primary endpoint, “How was the quality of your sleep last night?” showed a statistically significant difference in change from baseline in favour of VapoRub treatment (p = 0.0392) versus placebo. Positive effects of VapoRub versus placebo were also observed for “How refreshed did you feel upon waking up?” (p = 0.0122) (SQSQ), “Did you get enough sleep?” (p = 0.0036) (KSD), “How was it to get up?” (p = 0.0120) (KSD) and “Do you feel well-rested?” (p = 0.0125) (KSD). No statistically significant changes from baseline versus placebo were detected in the Actiwatch endpoints. Vicks VapoRub® when applied before retiring to bed can reduce subjective sleep disturbances during a common cold. The results of this exploratory study support the belief among patients that the use of VapoRub improves subjective sleep quality during common cold which was associated with more refreshing sleep.
Keywords:
Upper Respiratory Tract Infection, Common Cold, VapoRub, Sleep Disturbance, Aromatic Oils

1. Introduction
The common cold, an infection of the upper respiratory tract, is reported to be the most common human infectious disease [1] [2] . Adults can experience two to four episodes a year and children six to eight [2] .
Common cold is generally a mild illness of the upper respiratory tract, primarily affecting the nose, nasopharynx and paranasal sinuses and is readily self-diagnosed by sufferers [3] . Rhinoviruses are the most common causative agents accounting for up to 50% of symptomatic infections [1] . The main symptoms of common cold include nasal congestion, nasal discharge, sneezing, headache, sore throat, and cough [4] . Of these, nasal congestion and cough have been reported as the most bothersome symptoms of a cold on 6 of the first 7 days of a cold [5] .
Additionally, it is well recognised that during symptomatic common cold, sleep can be adversely affected [6] . While this effect may be considered modest (c. 23 minutes decrease in sleep and a 5% reduction in sleep efficiency) from a scientific perspective [7] , it is generally accepted that common cold-induced sleep disruption is clinically meaningful. Smith (2012) [6] showed correlations between symptom scores and sleep parameters. In general sleep measures and total symptom score were correlated, indicating that increasing symptom severity was associated with sleep disturbance. The data suggested that nasal congestion severity was a significant driver of the correlation. Therefore, remedies that can alleviate rhinitis induced disturbances of sleep without the use of sedation, to ensure a well-rested feeling upon awakening, have an important place in therapy.
In the UK and many other countries, Vicks VapoRub® (VVR) is indicated for the reduction in cough frequency [8] and feeling of relief from nasal congestion [9] . As cough and nasal congestion (regardless of aetiology) are recognised barriers to restful and restorative sleep [10] , [11] , [12] , their relief using a topical ointment like VVR can be predicted to improve elements of sleep quality.
VVR is a pharmaceutical preparation containing a combination of levomenthol (2.75% w/w), eucalyptus oil (1.5% w/w), turpentine oil (5% w/w) and camphor (5% w/w) as active ingredients, and thymol, cedarwood oil, and white soft paraffin (petrolatum) as excipients. VVR is an ointment that is either applied topically to the chest, throat, and back or added to hot water and the aromatic vapours inhaled. When applied to the skin, the active ingredients are evaporated by body temperature and inspired into the airways. The therapeutic effects, reduction in cough frequency and relief from nasal congestion, are likely due, at least in part, to interactions of the aromatics with the largely calcium-selective ion channel, transient receptor potential (TRP) receptors. Recent data suggest that the transient receptor potential receptors TRPM8, TRPV1 and TRPA1 are up-regulated in respiratory virus infected cells [13] [14] . TRPM8 mediates the feeling of coolness associated with menthol and eucalyptus oil [15] , [16] and so is likely the main mediator of the sensation of cooling and nasal decongestion associated with menthol and eucalyptus oil [9] . Camphor, eucalyptus oil and menthol have been shown to interact with the TRPV1 and TRPA1 receptors [17] [18] [19] which are implicated in the neurophysiology of cough [13] [14] . These interactions may therefore have a role in controlling cough sensitivity.
This study compared the effects of VVR versus placebo on subjectively and objectively assessed sleep parameters in adult common cold sufferers. Sleep parameters were measured using subjective questionnaires (Subjective Quality of Sleep Questionnaire [SQSQ] [20] , a modified Karolinska Sleep Diary [KSD] [21] , and a study-specific post-sleep questionnaire) and actigraphy, an objective method of monitoring rest-activity patterns [22] . Several subjective questionnaires were employed because this was the first exploration of the effect of VVR on sleep parameters so little knowledge existed of likely effect size or which elements of sleep may be affected. Actigraphy was employed as it is a validated objective albeit surrogate measure of sleep.
2. Methods
2.1. Participants
One hundred and forty-one adult participants were screened for this study and 100 were enrolled and randomized to the 2 treatment groups (Figure 1). The mean (SD) age of those enrolled was 23 years (8.7) with a mean Body Mass Index (BMI) of 23 (2.9). The population was 61% female and most participants (71%) were Caucasian. One participant withdrew consent during the study and their data were not included. Subjects were recruited by advertisement from the staff and students of the University of Surrey and the greater London area. Those participating received £120 compensation.
Randomized participants were suffering from a common cold and experiencing nasal congestion, cough and disturbance to normal sleep. Key inclusion criteria included suffering from a self-diagnosed common cold of no more than 36 hours duration; suffering from at least mild cough and nasal congestion due to the common cold (scores of at least 1 on the 4-point ordinal scale); having an average score of < 50 on the 2 responses to the question “How would you compare the quality of last night’s sleep with your usual sleep without a cold?” from the Leeds Sleep Evaluation Questionnaire (LSEQ) [23] , a 100-mm Visual Analog Scale (VAS), where Response 1 is 0 = “Less restful than usual” and 100 = “More
Figure 1. Subject disposition.
restful than usual” and Response 2 is 0 = “More periods of wakefulness than usual” and 100 = “Fewer periods of wakefulness than usual”.
Volunteers were excluded if they had any of the following: A previously diagnosed sleep disorder, a current sleep disturbance or poor sleep quality unrelated to their cold based on the Pittsburgh Sleep Quality Index (PSQI) [24] (i.e., a score of >5); a clinically significant nasal abnormality; a history of clinically relevant anosmia; were employed on night or rotating shift work or needed to travel across more than 2 time zones in the 14 days prior to screening or planned to do so during the study; a history of allergy or hypersensitivity to any of the ingredients of VVR; a history of significant airway disease or pronounced hypersensitivity of the airways/asthma or Chronic Obstructive Pulmonary Disease, a significant history of recurrent sinusitis or currently experiencing allergic rhinitis, or significant history of chronic cough; a body temperature > 100.5°F (38.1˚C); had used, within 5 half-lives, substances or medications known to affect sleep; had used nasal decongestants in the past 24 hours; had a self-reported consumption of >5 caffeinated beverages daily; used nicotine in any form; took naps daily; used inhaled, topical, or oral nedocromil or cromolyn sodium, tricyclic antidepressant medications, or monoamine oxidase inhibitors for 14 days prior to screening; had a history of alcohol or drug abuse within the past 2 years; were currently enrolled in another clinical trial, or had received any other investigational drug within the past 30 days; if female and of child-bearing potential had a positive urine pregnancy test at screening or were lactating; had a history of malignancy within the past 2 years, or had current or past history of serious, severe, or unstable physical or psychiatric illness; or were taking medication that the Investigator believed would interfere with the evaluation of the study, pose a safety risk, or confound the interpretation of the study results.
2.2. Study Design
The study (EudraCT# 2013-004524-11) was a randomized, single-(Investigator) blind, controlled, 2-arm (Vicks VapoRub® [VVR] vs. petrolatum), parallel design, single site study conducted at Surrey Clinical Research Centre, University of Surrey, between November 2014 and May 2015.
The study was conducted in accordance with the ICH Guideline for Good Clinical Practice, 1997; the US CFR Title 21 parts 50, 56 and 312; applicable national laws and regulations; the ethical requirements of Directive 2001/20/EC; and the ethical principles with their origin in the Declaration of Helsinki. The study was approved by the NRES Committee London-Brent Ethics Committee and all participants provided written informed consent prior to any study procedures being conducted.
The study included a baseline visit (Day 0) at the study site to confirm study eligibility and to randomly assign subjects to 1 of 2 test products (VVR or petrolatum). Randomized subjects were sent home with the SQSQ, KSD, and post- sleep questionnaire, along with the Actiwatch sleep monitoring equipment and their assigned test product. Subjects were instructed to use the Actiwatch on this first evening of Day 0, but did not apply test product. Upon waking the next morning, subjects completed the SQSQ, KSD, and the post-sleep questionnaire.
The test period began on the evening of Day 1 when subjects applied their randomly assigned test product as directed, immediately before going to bed. Subjects continued using the Actiwatch overnight. Upon waking the next morning, subjects completed the SQSQ, KSD, and post-sleep questionnaire. These procedures were repeated on the evening of Day 2 and the following morning. Subjects then returned to the study site on Day 3 to complete exit procedures and to return their sleep monitoring equipment, completed questionnaires, and test product containers with any remaining study test product. At this point, the subjects exited from the study.
2.3. Test Products
7.5 grams of commercially available VVR and petrolatum base (placebo) were packaged in identical individual 25 gram jars identifiable only by participant number. Participants were provided with 2 identical jars of either VVR or placebo at Day 0 with the instructions to apply all of the product from the first jar on the evening of Day 1 at bedtime, and all of the product from the second jar on the evening Day 2 at bedtime.
2.4. Sleep Measures
The effect size of VVR on sleep was not expected to be large in absolute terms. Further, we were most interested in the subjective perceptions of sleep quality therefore change in the, “How was the quality of your sleep last night?” question of the SQSQ, a validated questionnaire which has been shown to be sensitive to effects of zolpidem, temazepam [25] , gaboxadol and traffic noise [20] and slow wave sleep disruption by acoustic stimuli [26] , was chosen as the primary endpoint. The KSD questionnaire [21] , [27] was modified for this study by removing the first 5 questions due to duplication of sleep measures with the SQSQ.
Actigraphy is considered a valid method to quantify sleep patterns in healthy controls, patients with sleep disorders and their treatment response [28] . This method was included in the study because if positive correlation with the subjective measures were observed, it would provide additional confidence in the effect and its magnitude. The Actiwatch 4® is a gyroscopic actigraphic device worn on the non-dominant wrist to collect objective indirect measurements of sleep and wakefulness (i.e., where movement is a surrogate for wakefulness) utilizing an automated computer and scoring algorithm. Derived sleep measures were total sleep time, sleep onset latency, mean activity score, percentage of sleep (percentage of actual sleep time between sleep onset and sleep end), sleep efficiency (percentage of time spent asleep from “Lights out” to “Lights on”), and number of sleep bouts. For computations of sleep and wakefulness the software algorithm used the activity data recorded by the Actiwatch 4® in a series of linked calculations, such that each data point from each epoch and those surrounding was used to compute a total score based on these activity counts. With a default Medium Sensitivity, for 1-minute epoch data (and pro rata for other epochs used) a total score of 40 was designated as an “Awake” epoch. The activity scale was set to 2000. To determine “Sleep Start”, the algorithm looked for a period of at least 10 minutes of consecutively recorded immobile data, with no more than 1 epoch of movement within that time, following the “Bed Time”. To determine ‘Sleep End’, the algorithm looked for a 10-minute consecutive period of activity around the “Get Up Time” and then worked back to find the last epoch of immobility. To set the analysis window, the actigraphy marker-based bedtime and get-up times were used. In instances, where subjects failed to use the markers, their sleep-diary based bedtime and get up times were used instead.
2.5. Statistical Methods
Randomization and Stratification of Participants: All potential study participants were given a subject number (starting at 1001 in the order in which they were screened for the study). Eligible volunteers were stratified by average LSEQ score on the 2 responses to the question “How would you compare the quality of last night’s sleep with your usual sleep without a cold?” (0 - 20.8 = “Very poor”; > 20.8 - 35.4 = “Rather poor”; > 35.4 - < 50 = “Intermediate”). Participants were then randomly assigned to test products (VVR or placebo) using a block randomization. A unique randomization number (e.g., 101, 102, 103, etc) was assigned to each eligible participant.
Safety Analyses: All safety summarization was done on Intent-to-Treat population (all randomized subjects).
Efficacy Analyses: All efficacy analyses were done on Per-Protocol population. The Per-Protocol population comprised those participants who were generally compliant with test product usage instructions (used ≥ half of the allocated dose) and met key inclusion and exclusion criteria. The Per-Protocol assessment was determined on blinded data prior to receiving treatment codes. In order to obtain a more consistent response, the two treatment days (Day 1 and Day 2) for each endpoint were averaged for analysis purposes and served as the response variable for the analyses.
Comparability of treatments at baseline for demographics, baseline characteristics, SQSQ, post-sleep questionnaire, Actiwatch, and KSD was assessed via 2- sample t-test, Fisher’s exact test, and Cochran-Mantel-Haenszel test, as appropriate per the data type. Analysis of covariance (ANCOVA) was used for analyzing primary and secondary endpoints using the Mixed procedure of SAS version 9.4 (SAS Institute, Cary, NC, USA).The primary endpoint was sleep quality as measured by the SQSQ. Each hypothesis was tested separately using an ANCOVA model that included relevant baseline measures as a covariate and treatment group as independent variable (fixed factor). The following hypotheses were tested separately for each endpoint:
Null Hypothesis: the change from baseline mean
Two of the secondary KSD measures (“Did you take any drugs before retiring?” and “Did you wake up ahead of time without being able to return to sleep?”) required nonparametric assessments as described in the protocol due to assumptions of ANCOVA not being met. All hypotheses were tested at a two-sided significance level of 5%. No corrections for multiplicity were conducted in this exploratory investigation.
2.6. Sample Size
The sample size was determined by logistical considerations and previous experience with sleep studies.
3. Results
Demographics: Table 1 shows the demographic composition of the participants.
Participant screening and baseline characteristics: common cold symptom severity, PSQI, LSEQ (abbreviated, only displayed two Leeds questions that were part of inclusion criteria), SQSQ, KSD and Post-sleep questionnaire, did not differ between groups (Tables 1-3).
Product Dosing Compliance: Product dosing compliance was assessed by weighing the sample jars before and after treatment. On average participants used 23% less product than instructed on both treatment nights. There were no
Table 1. Summary of demographics and baseline characteristics (Intent-to-treat).
Values are means (SD) or n(%) of subjects. 1P-values were calculated with 2 sample t-test for continuous variables, Fisher's exact test for non-ordered categorical variables, and Cochran-Mantel-Haenszel test for ordered categorical variables. Continuous data that violated normality were also analyzed nonparametrically with the Wilcoxon Rank Sum Test with similar conclusions of no statistically significant difference (p > 0.05). 2Pittsburgh Sleep Quality Index. Leeds sleep evaluation questionnaire question: “How would you compare the quality of last night’s sleep with your usual sleep without a cold?” 3A 100-mm Visual Analog Scale (VAS), where response is 0 = “Less restful than usual” and 100 = “More restful than usual”. 4A 100-mm Visual Analog Scale (VAS), where response is 0 = “More periods of wakefulness than usual” and 100 = “Fewer periods of wakefulness than usual”.
Table 2. Summary of subjective quality of sleep questionnaire, post-sleep questionnaire, and actiwatch for baseline (per-protocol).
Values are means (SD). 1P-values were calculated with 2-sample t-test. Continuous data that violated normality were also analyzed nonparametrically with the Wilcoxon Rank Sum Test with similar conclusions of no statistically significant difference (p > 0.05). 2VAS with lower numbers better. 3VAS with higher numbers better.
between-group differences―both groups used between 5.7 and 5.8 grams versus the supplied single-dose amount of 7.5 grams. The Per-Protocol analyses excluded seven participants for the nights they used less than half the allocated dose (3 VVR and 4 placebo participants).
Table 4 shows the data from statistical testing of between group differences in the various subjective scales used.
Subjective Quality of Sleep Questionnaire Endpoints: Statistical testing of the between group differences showed that the primary endpoint of Sleep Quality in the SQSQ showed a statistically significant difference in change from baseline in favour of VVR treatment compared to placebo (Table 4, Figure 2; p = 0.0392). This was also found for the “Wake up refreshed” SQSQ endpoint (Table 4, Figure 2; p = 0.0122). None of the other SQSQ endpoints showed statistically significant between group differences (Table 4, Figure 2). Examination of the monadic changes from baseline were consistent with the between group findings: 89% of participants who used VVR reported an improved quality of sleep vs
Table 3. Summary of Karolinska sleep diary for baseline (Per-Protocol).
N = number of subjects within specified treatment. n(%) = number and percentage of subjects within specified parameter, treatment, and category. 1P-values were calculated with Fisher’s exact test and Cochran-Mantel-Haenszel test, as appropriate per the data type.
Table 4. Analysis of covariance of subjective sleep questionnaires and diaries (Per-Protocol).
1. 0 - 100 VAS scale where lower values are better (negative treatment difference better); 2. 0 - 100 VAS scale where higher values are better (positive treatment difference better).
baseline, and 91% who used VVR reported an improved “wake up refreshed” score vs baseline. In examining the consistency of response of the primary endpoint (sleep quality as measured by SQSQ), subgroup analyses by demographics and baseline characteristics were assessed. The results for all subgroups analysed favoured VVR compared to placebo, indicating consistency of response. Statistical significance of VVR vs. placebo for sleep quality was observed in the subgroup of subjects with moderate-to severe cough at baseline (p = 0.0294), runny nose not present/mild at baseline (p = 0.0492) and PSQI ≥ 3 (p = 0.0486) (Data not shown).
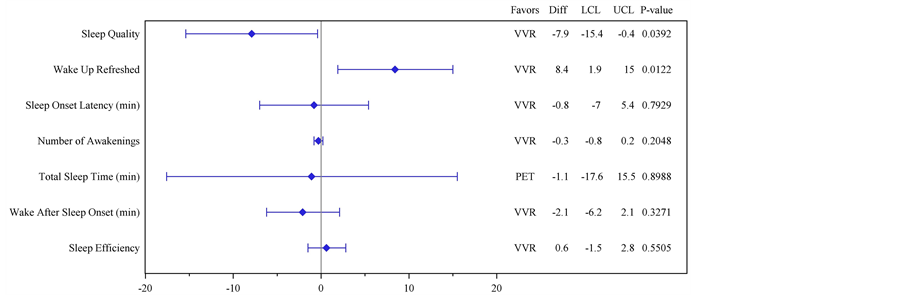
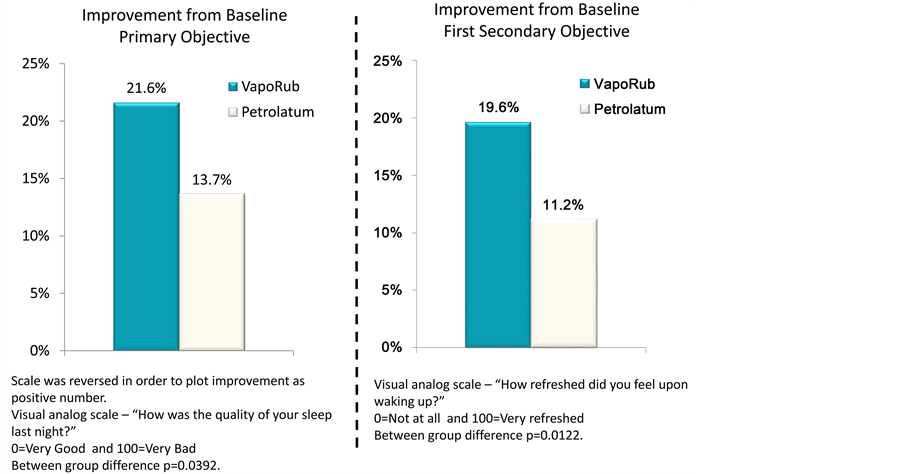
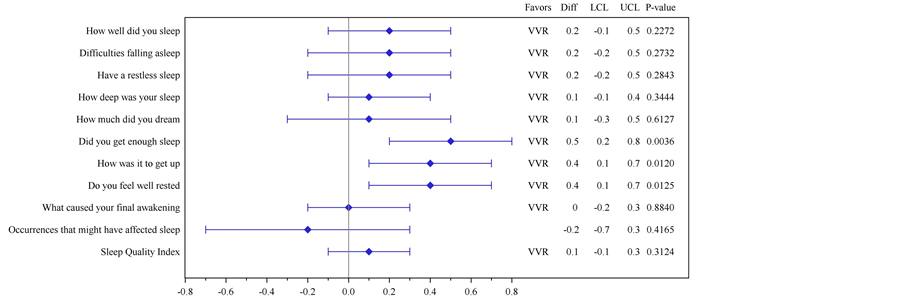
Figure 2. (a) Plot of mean differences and 95% Confidence Intervals for each of the Subjective Quality of Sleep Questionnaire (SQSQ) questions comparing answers provided “on awakening” with baseline; (b) Primary. Histogram showing the statistical analysis of results from the SQSQ question identified a piori as Primary Objective comparing answers provided “on awakening” with baseline. Visual Analogue Scale?“How was the quality of your sleep last night?” 0 = “Very Good” and 100 = “Very Bad”. Between group difference statistically significant at p < 0.0392. (b) Secondary. Histogram showing the statistical analysis of results from the SQSQ question identified a piori as “First Secondary Objective”, comparing answers provided “on awakening” with baseline. Visual Analogue Scale?“How refreshed did you feel upon waking up?” 0 = “Very Good” and 100 = “Very Bad”. Between group difference statistically significant at p < 0.0122.
Karolinska Sleep Diary Endpoints: Statistical testing of the KSD endpoints showed significant between group differences for “Did you get enough sleep?” (p = 0.0036), “How was it to get up?” (p = 0.0120) and “Do you feel well-rested?” (p = 0.0125) favouring VVR compared to placebo (Table 4, Figure 3). While none of the other KSD or Post-sleep Questionnaire endpoints reached statistical significance, VVR treatment showed a numerical improvement in the KSD results (Table 4 & Figure 3). The two secondary KSD measures (“Did you take any
Figure 3. Plot of mean differences and 95% Confidence Intervals for each of the Karolinska Sleep Diary (KSD) questions comparing answers provided “on awakening” with baseline.
drugs before retiring?” and “Did you wake up ahead of time without being able to return to sleep?”) analyzed by nonparametric measures did not show significant treatment differences (data not shown). A trend in favour of VVR compared to placebo (p = 0.0606), was observed in change from baseline for the “How deep was sleep?” endpoint in the Post-sleep Questionnaire but no difference in ease of falling asleep (Table 4).
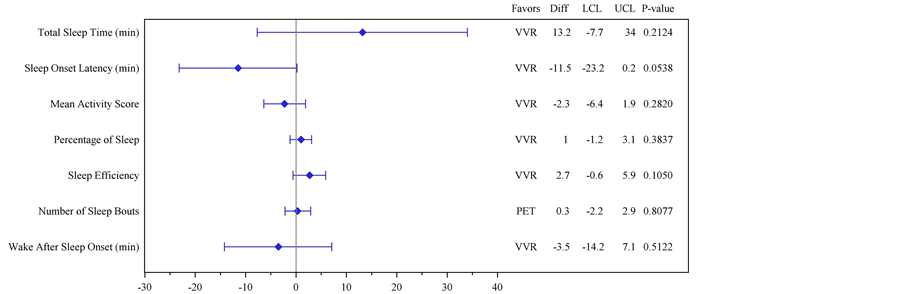
Actigraphy Endpoints: There were no statistically significant changes from baseline treatment differences in the Actiwatch endpoints. There was, however, a trend in favour of VVR compared to placebo for change from baseline in Sleep Onset Latency (Table 5, Figure 4; treatment difference 11.5 minutes and p = 0.0538).
Safety
Both treatments were well-tolerated during this study. There were no deaths, serious or other significant Adverse Events (AEs). Eight participants reported 10 AEs; 5 on placebo and 3 on VVR and all resolved without any action being taken to ameliorate the AE.
4. Discussion
The results from this exploratory study show for the first time that application of Vicks VapoRub® before retiring to bed has a positive effect on perceived sleep quality for adults suffering from symptoms of common cold. The primary endpoint subjective finding supports the long-held association described by common cold patients of VVR use and improved sleep. It cannot be determined from these data alone whether this is a result of symptom relief facilitating sleep (as has been suggested for cough relief [29] ) or a direct effect on sleep. However, the active ingredients in VVR are not known to have a sedative effect. This suggests that a patient-perceived sleep quality benefit in patients with a cold would be due to a different mechanism, likely symptom relief. Indeed, the subgroup of patients with moderate-to-severe cough at baseline reported improvement for sleep quality vs placebo (p = 0.0294).
Table 5. Analysis of covariance of Actiwatch (Per-Protocol).
Figure 4. Plot of mean differences and 95% Confidence Intervals for each of the Actiwatch endpoints comparing answers provided on awakening with those at baseline.
It was hypothesised that VVR would affect sleep as a result of its known decongestion and antitussive effects rather than as a sleep aid. Consequently, while for completeness both subjective and objective assessments of sleep were employed, it was predicted that subjective endpoints were most likely to be affected. Change in the SQSQ sleep quality was chosen as the primary objective as it offered a global retrospective assessment of the perceived sleep experience. The KSD was also included as a source of secondary endpoints to permit a more granular description of the subjective elements of sleep affected. As nasal decongestion and cough relief are the licensed indications of VVR in the UK, they were not measured in this study wherein the focus was sleep-related effects.
The difference in perceived sleep quality assessed by the SQSQ was statistically significant (p = 0.0392). To place this change in perspective it is noted that the observed response to VVR compared to placebo for the SQSQ was 7.9 units, which is comparable to the positive effects of gaboxadol on subjective sleep quality in a traffic noise model of sleep disruption [20] and approximately half the size of the effect of zolpidem and temazapam on sleep in middle-aged people [25] . Supporting this is the finding that responses to the “How refreshed did you feel upon waking?” SQSQ endpoint were also significant (p = 0.0122). On average subjects had 58% better sleep quality vs. placebo and 75% better wake up refreshed vs. placebo. The effect sizes for questions [30] would be considered a moderate effect (0.44 and 0.54 respectively). Taken together these findings suggest that VVR had a clinically relevant effect on the perception of sleep quality. We speculate that as subjective sleep assessments are necessarily retrospective, the fact that the VVR aroma would still be noticeable in the morning may have augmented the perception of improved sleep quality.
The SQSQ also includes a series of more specific time and duration related questions. Changes in these parameters did not reach statistical significance, consistent with the objective actigraphy results except for sleep onset latency which objectively favoured VVR with subjects falling asleep approximately 11.5 minutes faster than placebo (p = 0.0538). Arguably, questions around for example, time to get to sleep, number of awakenings, duration of awakenings, etc. require an awareness of sleep and sleep disruption that may be difficult to measure in patients with an illness such as the common cold relative to other conditions; further studies would be useful. It seems reasonable to expect greater effects if subjects had more severe symptoms and/or more severe sleep disturbance at baseline.
There were also positive findings for some of the secondary endpoints assessed by the KSD. Participants provided positive (for VVR) between group responses for, “Did you get enough sleep?” (p = 0.0036), “How was it to get up?” (p = 0.0120) and “Do you feel well-rested?” (p = 0.0125). The effect size for the significant KSD questions (0.62, 0.53, and 0.53 respectively) would be considered moderate effects [30] . These parameters may be considered to be elements of the overall findings of improved sleep quality and the feelings of having had refreshing sleep. They are consistent with improvements in an improved overall sleep experience. The remaining KSD parameters including, “How did you sleep?” and sleep quality index, did not show a statistical significant effect.
The objective effects on sleep were assessed using arm motion during rest as a surrogate measure for wakefulness. The Actiwatch instrument has been validated for the assessment of a variety of sleep parameters [28] [31] . We believe this study is the first use of actigraphy in the investigation of the mild sleep disturbance associated with common cold. The Actiwatch and subjective data were broadly consistent. In this study of sleep disturbance associated with common cold, no significant between-group differences were detected in the objective data-only “Sleep Onset Latency” approached statistical significance (p = 0.0538).
Several limitations of this study merit discussion. An obvious limitation is that it was not double-blind. As the Informed Consent information made clear that participants would be treated either with an aromatic or non-aromatic product, those allocated VVR would have been aware that they had received active product as soon as they sensed the aroma. The difficulty in designing double-blind studies with VVR is a recognized design limitation [32] . It is difficult to blind a study wherein the aroma of the product is so widely recognized and represents the pharmacological effect. Investigators were blinded as no medication was opened at the study site thus preserving the single blind.
Finally, while these data indicate a positive effect of VVR on sleep quality they cannot distinguish between VVR treatment improving sleep quality solely as a result of reducing the symptoms, exerting a direct sleep effect or a combination of both. Further work is recommended wherein the direct sleep quality benefit in persons with sleep disturbance and no infectious disease is examined.
5. Conclusion
Common cold sufferers using Vicks VapoRub® (applied before retiring to bed) experienced significant differences in self-reported sleep quality compared to control. This supports that treatment with this product can be a valuable component in the armamentarium of safe and effective common cold therapies due to the patient perceived improvement in sleep quality, a widespread patient belief measured in this study.
Financial and Competing Interests
This work was sponsored in full by Procter & Gamble. At the time of conducting the study and preparing this manuscript, DH, GP and DR were full-time employees of The Procter & Gamble Company and may have stock and/or stock options in the company. VR, NS and DJD did not receive any financial payments or other inducement apart from their usual university salary, for conducting this study.
DH and DR were responsible for statistical analysis (DR) interpretation of study results (DH, DR) and publication drafting (DH). VR, NS and DJD were responsible for study execution. GP advised on design and was responsible for product supply and management. All attributed authors participated in the development and review of this manuscript.
Acknowledgements
The authors gratefully acknowledge the assistance of J. Brum, G. Kappler and P. Thomas of Procter & Gamble in study design and for excellence in protocol development and study management. Also acknowledged is the expert advice of Ian Barton (Procter & Gamble) during study design.
Thanks also to the Clinical Research and Medical teams at the Surrey Clinical Research Centre.
Cite this paper
Santhi, N., Ramsey, D., Phillipson, G., Hull, D., Revell, V.L. and Dijk, D.-J. (2017) Efficacy of a Topical Aromatic Rub (Vicks VapoRub®) on Effects on Self-Reported and Actigraphically Assessed Aspects of Sleep in Common Cold Patients. Open Journal of Respiratory Diseases, 7, 83-101. https://doi.org/10.4236/ojrd.2017.72009
References
- 1. Wat, D. (2004) The Common Cold: A Review of the Literature. European Journal of Internal Medicine, 15, 79-88.
- 2. Heikkinen, T. and Järvinen, A. (2003) The Common Cold. The Lancet, 361, 51-59.
- 3. Eccles, R. (2013) Is the Common Cold a Clinical Entity or a Cultural Concept? Rhinology, 51, 3-8.
- 4. Eccles, R. (2005) Understanding the Symptoms of the Common Cold and Influenza. The Lancet Infectious Diseases, 5, 718-725.
- 5. Witek, T.J., et al. (2015) The Natural History of Community-Acquired Common Colds Symptoms Assessed over 4-Years. Rhinology, 53, 81-88. https://doi.org/10.4193/Rhin14.149
- 6. Smith, A.P. (2012) Sleep and the Common Cold. Journal of Behavioral Health, 1, 114-117.
https://doi.org/10.5455/jbh.20120322073850 - 7. Drake, C.L., et al. (2000) Effects of an Experimentally Induced Rhinovirus Cold on Sleep, Performance, and Daytime Alertness. Physiology & Behavior, 71, 75-81.
- 8. Packman, E.W. and London, S.J. (1980) The Utility of Artificially Induced Cough as a Clinical Model for Evaluating the Antitussive Effects of Aromatics Delivered by Inunction. European Journal of Respiratory Diseases, 110, 101-109.
- 9. Eccles, R., et al. (2015) Efficacy of a Topical Aromatic Rub (Vicks VapoRub®)-Speed of Action of Subjective Nasal Cooling and Relief from Nasal Congestion. Open Journal of Respiratory Diseases, 5, 10-18.
https://doi.org/10.4236/ojrd.2015.51002 - 10. Meltzer, E.O. (2002) Does Rhinitis Compromise Night-Time Sleep and Daytime Productivity? Clinical & Experimental Allergy Reviews, 2, 67-72.
https://doi.org/10.1046/j.1472-9725.2002.00039.x - 11. Dicpinigaitis, P.V., et al. (2009) Acute Cough: A Diagnostic and Therapeutic Challenge. Cough, 16, 11. https://doi.org/10.1186/1745-9974-5-11
- 12. Sabharwal, G. and Craig, T.J. (2016) The Effect of Rhinitis on Sleep, Quality of Life, Daytime Somnolence, and Fatigue. In: Mahmoudi, M., Ed., Allergy and Asthma, Springer International Publishing, Berlin, 87-97. https://doi.org/10.1007/978-3-319-30835-7_7
- 13. Omar, S., et al. (2017) Respiratory Virus Infection Up-Regulates TRPV1, TRPA1 and ASICS3 Receptors on Airway Cells. PLoS ONE, 12, e0171681.
- 14. Abdullah, H., et al. (2014) Rhinovirus Upregulates Transient Receptor Potential Channels in a Human Neuronal Cell Line: Implications for Respiratory Virus-Induced Cough Reflex Sensitivity. Thorax, 69, 46-54. https://doi.org/10.1136/thoraxjnl-2013-203894
- 15. McKemy, D.D., et al. (2002) Identification of a Cold Receptor Reveals a General Role for TRP Channels in Thermosensation. Nature, 416, 52-58. https://doi.org/10.1038/nature719
- 16. Bautista, D.M., et al. (2007) The Menthol Receptor TRPM8 Is the Principal Detector of Environmental Cold. Nature, 448, 204-208. https://doi.org/10.1038/nature05910
- 17. Xu, H., et al. (2005) Camphor Activates and Strongly Desensitizes the Transient Receptor Potential Vanilloid Subtype 1 Channel in a Vanilloid-Independent Mechanism. Journal of Neuroscience, 25, 8924-8937. https://doi.org/10.1523/JNEUROSCI.2574-05.2005
- 18. Juergens, U.R. (2014) Anti-Inflammatory Properties of the Monoterpene 1.8-Cineole: Current Evidence for Co-Medication in Inflammatory Airway Diseases. Drug Research, 64, 638-646. https://doi.org/10.1055/s-0034-1372609
- 19. Caceres, A.I., et al. (2017) Transient Receptor Potential Cation Channel Subfamily M Member 8 Channels Mediate the Anti-Inflammatory Effects of Eucalyptol. British Journal of Pharmacology, 174, 867-879. https://doi.org/10.1111/bph.13760
- 20. Dijk, D.-J., et al. (2012) Enhanced Slow Wave Sleep and Improved Sleep Maintenance after Gaboxadol Administration during Seven Nights of Exposure to a Traffic Noise Model of Transient Insomnia. Journal of Psychopharmacology, 26, 1096-1107.
https://doi.org/10.1177/0269881111421971 - 21. Akerstedt, T., et al. (1994) The Subjective Meaning of Good Sleep, an Intra-Individual Approach Using the Karolinska Sleep Diary. Perceptual and Motor Skills, 79, 287-296.
https://doi.org/10.2466/pms.1994.79.1.287 - 22. Sadeh, A. (2011) The Role and Validity of Actigraphy in Sleep Medicine: An Update. Sleep Medicine Reviews, 15, 259-267.
- 23. Parrott, A.C. and Hindmarch, I. (1980) The Leeds Sleep Evaluation Questionnaire in Psychopharmacological Investigations—A Review. Psychopharmacology, 71, 173-179.
https://doi.org/10.1007/BF00434408 - 24. Buysse, D.J., et al. (1989) The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Research, 28, 193-213.
- 25. Arbon, E.L., et al. (2015) Randomised Clinical Trial of the Effects of Prolonged-Release Melatonin, Temazepam and Zolpidem on Slow-Wave Activity during Sleep in Healthy People. Journal of Psychopharmacology, 29, 764-776. https://doi.org/10.1177/0269881115581963
- 26. Dijk, D.J., et al. (2010) Age-Related Reduction in Daytime Sleep Propensity and Nocturnal Slow Wave Sleep. Sleep, 33, 211-223.
https://doi.org/10.1093/sleep/33.2.211 - 27. Åkerstedt, T., et al. (1994) The Meaning of Good Sleep: A Longitudinal Study of Polysomnography and Subjective Sleep Quality. Journal of Sleep Research, 3, 152-158.
https://doi.org/10.1111/j.1365-2869.1994.tb00122.x - 28. Morgenthaler, T., et al. (2007) Practice Parameters for the Use of Actigraphy in the Assessment of Sleep and Sleep Disorders: An Update for 2007. Sleep, 30, 519-529.
https://doi.org/10.1093/sleep/30.4.519 - 29. Cohen, H.A., et al. (2012) Effect of Honey on Nocturnal Cough and Sleep Quality: A Double-Blind, Randomized, Placebo-Controlled Study. Pediatrics, 130, 465-471.
https://doi.org/10.1542/peds.2011-3075 - 30. Cochrane Organisation (2017).
http://handbook.cochrane.org/chapter_12/12_6_2_re_expressing_smds_using_rules_of_thumb_for_effect_sizes.htm - 31. Ancoli-Israel, S., et al. (2003) The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep, 26, 342-392. https://doi.org/10.1093/sleep/26.3.342
- 32. Paul, I.M., et al. (2010) Vapor Rub, Petrolatum, and No Treatment for Children with Nocturnal Cough and Cold Symptoms. Pediatrics, 126, 1092-1099. https://doi.org/10.1542/peds.2010-1601