Open Journal of Genetics
Vol.1 No.2(2011), Article ID:7315,9 pages DOI:10.4236/ojgen.2011.12005
Wolbachia induces sexual isolation in Drosophila melanogaster and Drosophila simulans
![]()
Department of Functional Biology, Faculty of Medicine, University of Oviedo, Oviedo, Spain.
Email: *ialahn@gmail.com; mcc@uniovi.es
Received 14 April 2011; revised 20 June 2011; accepted 30 July 2011.
Keywords: Wolbachia; Assortative Mating; Sexual Isolation; Speciation; Drosophila
ABSTRACT
Wolbachia are a group of intracellular bacteria, maternally transmitted from infected females to their offspring, which affect a wide range of arthropods. Their presence is associated with Cytoplasmic Incompatibility (CI) in crosses between infected males and uninfected females and between populations carrying different strains of Wolbachia. The negative influence of Wolbachia infection on progeny fitness in incompatible crosses can be considered a first step in the appearance of reproductive isolation between infected and uninfected individuals. In this work, we examined the possibility of assortative mating in response to Wolbachia infection, a response that evolved as an incipient mechanism of sexual isolation in the species D. melanogaster and D. simulans. We found that the females of each species could detect the presence of the bacterium in the other sex and chose to mate with males who had the same state of infection, whereas the males randomly attempted to mate with both infected and uninfected females. Thus, Wolbachia may act as an additive factor influencing sexual isolation in Drosophila populations and may play a role in speciation events.
1. INTRODUCTION
Species are groups of individuals that mate between themselves and share genes but are reproductively isolated from other populations and species. Speciation depends on the establishment of reproductive isolation between populations of the same species. Two mechanisms can establish reproductive isolation: postmating, which prevents gene flow between populations by progeny sterility and/or inviability, and premating, which prevents mating between individuals [1,2]. The genetic and environmental factors involved in the early stages of speciation remain poorly understood to evolutionary biologists.
Sexual isolation is a premating mechanism that occurs when individuals meet but do not mate. Generally, sexual isolation is total between remote species but partial between related species, at least under laboratory conditions. According to evolutionary theory, sexual isolation can be viewed as an incompatibility or disharmony between the sexual behaviour of two populations or species, a situation that favours homospecific over heterospecific matings (assortative mating).
Mating preferences are a consequence of genetic differences that ultimately affect male and female behaviour related to specific discrimination [3,4]. However, recent evidence has suggested that environmental factors may cause reproductive isolation between groups of the same species without a need for genetic changes, for example, infection by certain microorganism [5,6].
Wolbachia are intracellular, maternally transmitted bacteria that infect a broad range of invertebrates [7,8]; [9-12]. In arthropods Wolbachia manipulate their host’s reproduction in a variety ways, including cytoplasmic incompatibility (CI) [13], male killing [14], parthenogenesis induction [13,15], feminization of males [16]. These modifications of host reproduction impart a selective advantage for the bacteria [7]. Cytoplasmic Incompatibility (CI) [13,17]; is a sperm-egg incompatibility that results in embryonic death in crosses between infected males and uninfected females, or between animals infected with different strains of Wolbachia.
The bacteria can be eliminated by physical treatment (high temperature) or chemical (antibiotics such as tetracycline) treatment [18-21]. When the Wolbachia infection is removed from a population CI disappears, showing that the infection, and not solely genetic factors, is responsible for the negative fitness effects in incompatible crosses [22].
The elimination of bacteria by environmental factors could explain the coexistence of infected and uninfected individuals in some natural populations.
Several researchers have focused on the potential role of Wolbachia in speciation, due to the consequences of the infection in the postmating isolation of incompatible crosses [23-25,22]. However, up to now it is unknown if Wolbachia are involved in sexual isolation and mating discrimination between infected and uninfected individuals from the same population and species. The influence of the bacteria on mate choice has only detected in infected Drosophila melanogaster females [22], in uninfected spider mite females [26] and in Nasonia [27].
The main goal of this study was to examine if Wolbachia infection can cause sexual isolation between infected and uninfected individuals in the two sibling species, Drosophila melanogaster and D. simulans.
The genus Drosophila is good models which can be used to examine the possible effects of Wolbachia infection in the occurrence of speciation [28]. CI has been detected in several species with varying intensity [29,30], depending on the strain of Wolbachia [31-34] and the parasite-host genome interactions [35-39].
Drosophila melanogaster and D. simulans are cosmopolitan; they have a similar morphology and genetic backgrounds, but they are infected with different strains of Wolbachia [29,33]. Both show CI in incompatible crosses, although the effect is usually more severe in D. simulans than in D. melanogaster [30].
To test if Wolbachia infection is involved in the process of incipient speciation tests were carried out with female and male choice tests for each species. Sexual isolation performed with virgins (three days) infected and cured.
2. MATERIALS AND METHODS
Drosophila species
1) A population of Drosophila simulans captured in Sanabria (Spain) in 2003.
2) A spontaneous white mutant comes from the same population; the genetic background was made homogenous by nine backcrosses with the original wild flies. Both the wild and the white strains are infected with the wRi strain of Wolbachia.
3) A population of Drosophila melanogaster captured in Oviedo (Spain) in 1999.
4) A white strain created by crosses between wild females and white males strain (sent to us by the Bloomington centre). After nine backcrosses we obtained a White mutant strain which shares the same genetic background of D. melanogaster. Both the wild and the white strains are infected with the wMel strain of Wolbachia.
The experiments were conducted with the flies which stayed several years in the laboratory.
Before starting the experiment the presence of Wolbachia in both species was verified using PCR primers, corresponding to the 16S rDNA partial sequence of Wolbachia strains associated with Drosophila [40], and in this moment in our laboratory all the individuals of the both species are infected.
All lines and populations were reared in bottles with standard medium made of 100 g baker’s yeast, 100 g sugar, 12 g agar, 2 g salt and 5 ml propionic acid per litre of water.
Wolbachia were removed from files of both species by tetracycline treatment. The treatment was started with small concentration 0.25 g/l, the descendants of this generation was treated with 0.80 g/l and the new descendents from this concentration were put on medium with 1 g/l for three generations. After the treatment the new descendants were put on medium without tetracycline for at least three generations before experiments starting, to avoid the possible effect of the antibiotic on the flies’ fitness [21]. After the treatment all cured individuals lost the infection and that was confirmed by PCR.
The experiments were carried out with cured individuals come from 1 g/l of tetracycline. Individuals from the other concentrations were not used in the experiments.
Infected and cured flies of the both species were supported in separate culture chambers at 21.5˚C with 12 hour cycles of light and darkness.
To avoid inbreeding, the flies came from the progeny of twenty bottles with at least thirty pairs of parents in each bottle. The larval density was controlled, allowing egg laying for seven days, and then adults were removed. The flies were renewed each generation by randomly mass culture.
2.1. Experiment 1: Female Mating Propensity
Female mating propensity was estimated as the time elapsed from introduction to copulation with young virgin individuals (three days old). The mating speed of wild and white mutants, infected or cured, was assessed based on the reported importance of the propensity of the female in mating choice [41,42]. The female and male choices test can not be performed if there are great differences in mating propensity between infected and cured individuals.
The new emerged flies were kept in groups of ten males or females in isolated vials with food. Three days later, a male and a female were aspirated to a new vial without food, and the time elapsed before mating was recorded. The vial was observed until copulation occurred or up to a maximum of 30 minutes, and the number of pairs to estimate the female and male propensities in infected or cured and in wild and white individuals was about 50 (200 in each specie).
This experiment was carried out during a week for each species, every day in the morning from 10 to 14 hours, in a chamber with 21˚C constant temperature and artificial light, with 10 pairs from each phenotype and infection status (80 mating per day) randomly.
2.2. Experiment 2: Mutation Effects in Mating
To detect if the mutation has any effect on the choice possibility, female choice test was performed with infected females (wild or white) for each species.
It introduced a female with two males which differed in the eyes colour, all virgins’ individuals (three days old) from the same species and have same status of infection, in an empty vial of 20 cc. Then, observed the phenotype of mated individuals. The observation time was 40 minutes.
2.3. Experiment 3: Female and Male Choice Tests
The new emerged flies were kept in groups of ten males or females in isolated vials with food. Three days later, the Female choice experiments were carried out with young virgin (three days old) individuals. One female and two males (one infected and the other cured, with different phenotypes, a wild and a white), were placed in an empty vial glass to detect the phenotype of the successful male, the copulated pairs were kept under closed observation for a maximum of 45 minutes. Failures to copulate were discarded. Copulating pairs were examined to identify the type of the successful male.
The male choice experiments were done in a similar way; a male selects one of two females (one infected and the other cured, with different phenotypes, a wild and a white). Female and male choice test were carried out in the same generation of flies in each species.
The presence of Wolbachia was checked at the beginning and the end of each mating choice test. Both female and male choice tests were done for each pair-wise combination of strains and species. Around 400 data samples were obtained for each combination experiment.
The differences between mating numbers of individuals have the same status of infection (females and males either infected or cured) or have different status of infection (between infected and cured individuals) were estimated by a chi-square with a degree of freedom; the null hypothesis is equality of mating number, and by an isolation index (P) used by Prouzan [43].
This index is applicable to female and male choice tests. The index is the natural logarithm of the ratio of homogamic mating X11 (in our case corresponding to individuals with the same status of infection) and X12 heterogamic mating (corresponding to individuals with different status of infection, one infected and the other curd).

the standard error was calculated as:

If there is random mating P = 0.
The significance of the index was estimated by a t de Student with n – 1 degrees of freedom.
3. RESULTS
3.1. Experiment 1: Female Mating Propensity
The means and the standard errors of the time elapsed before mating (in seconds) and the results of the two ways ANOVA from both species of appear in Table 1. No significant differences were detected in D. simulans between normal or mutant phenotype individuals, infected or cured. Whereas some differences were detected in D. melanogaster between both phenotypes, the mating of wild individuals is faster than white counterparts, but the difference between the mating of the two phenotypes was too small to give any significance (11 minutes 4 seconds in the wild compared to 13 minutes and 27 seconds in white). Therefore, the possible effect of the presence of Wolbachia is not affected by the different mating propensity of both sexes.
3.2. Experiment 2: Mutation Effects in Mating
The males’ number of each phenotype chosen by females to mate is shown in Table 2. The comparison was estimated by a test of “Chi squared” with a degree of freedom. No differences were detected in the frequency of females mating with normal or mutant males in the both species due to the mutation.
3.3. Experiment 3: Female and Male Choice Tests
3.3.1. D. melanogaster
Female choice: The number of each male type chosen by a female to mate appears in Table 3 and total percentage of infected or cured males in each cross is shown in Figure 1. Comparison between the values by the chi-squared and by the isolation index (P) indicates that infected females mated more frequently with infected males, and cured females with cured males.
This phenomenon occurred independently of the female and male phenotype. The results indicate that females are able to detect the presence of Wolbachia in the opposite sex and mate with males who have same status of infection, and show assortative mating. The 86%

Table 1. D. simulans and D. melanogaster mating propensities. Mean time and standard error, in seconds, of the female and male mating propensities in the two species and strains, infected or cured (the number of pairs appear in parenthesis). The results of the two ways ANOVA from each species are listed below.

Figure 1. Percentage of infected and cured males mated with infected and cured females in female choice test.
(201/234) of infected females chose mating with infected males, and the 83% (199/238) of the cured females chose mating with cured males. The comparison between these two percentages by a contingency chi squared was (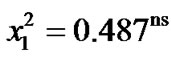 ), indicating that infected and cured females have the same discrimination in their choice mating.
), indicating that infected and cured females have the same discrimination in their choice mating.
Male choice: The number of each female type chosen by a male to mate appears in Table 4 and total percentage of infected or cured females in each cross is shown in Figure 2. As in previous tests, the frequency of different mating was compared by Chi squared and by the Isolation Index (P).
The results indicate that the males did not choose between both females infected or cured and show random mating.

Table 2. Number of each males phenotype selected by females in a female choice test. (All individuals were infected).
3.3.2. D. simulans
Female choice: The number of each male type chosen by a female to mate appears in Table 5 and total percentage of infected or cured males in each cross is shown in Figure 1. The differences between the mating frequencies were estimated by Chi squared and by the isolation index (P). The results of the comparison by both statistics indicated that the general behaviour of D. simulans females is alike D. melanogaster females.
The infected females chose mating with infected males in a 69% (162/235) and the cured females chose mating with cured males in a 68% (164/241). Both percentages are homogeneous ( = 0.04ns), infected and cured females discriminate in a similar way.
= 0.04ns), infected and cured females discriminate in a similar way.
Then, mating between pairs with an identical infection state occurred more frequently than the other possible combinations, and indicated assortative mating.

Table 3. D. melanogaster female choice. Number and comparison of males mating with females in combinations of infected and cured pairs.

Table 4. D. melanogaster male choice. Number and comparison of females mating with males in combinations of infected and cured pairs.
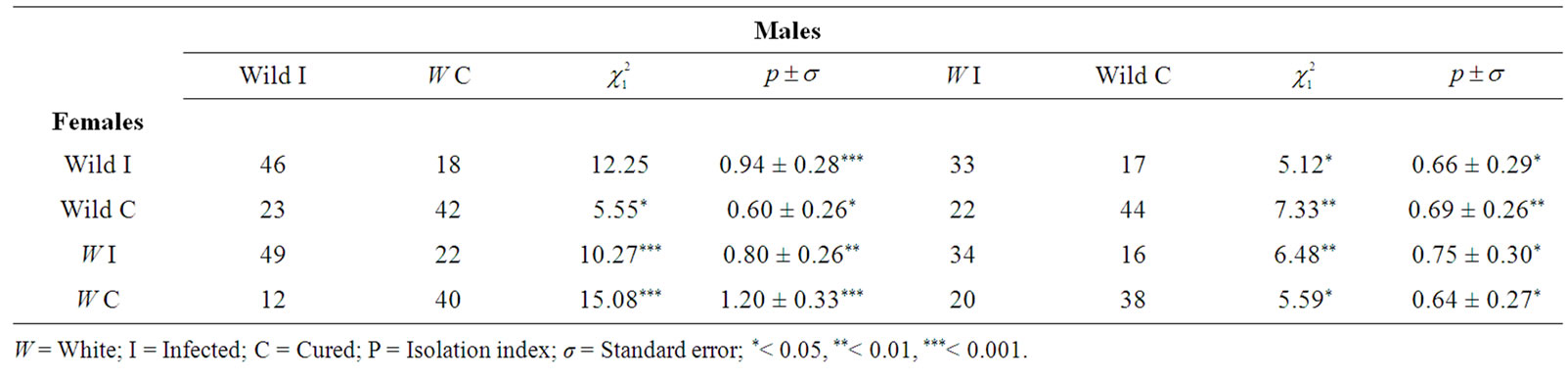
Table 5. D. simulans female choice. Number and comparison of males mating with females in combinations of infected and cured pairs.

Figure 2. Percentage of infected and cured females mated with infected and cured males in male choice test.
Male choice: The number of each female type chosen by a male to mate appears in Table 6 and total percentage of infected or cured females in each cross is shown in Figure 2. As in previous tests, the differences between the mating frequencies were estimated by Chi squared and by the Isolation Index (P). There was no significant difference in the mating frequency of males with either infected or cured females, indicated that the males did not choose between both types of females and show random mating, the same pattern observed in D. melanogaster males.
3.3.3. Comparison between Species
To detect which of the two species shows higher premating in the females’ choice tests, the results of infected and cured females were compared by a contingency chi square test. The total values appear in Table 7.
The results of the comparison indicated that the females of D. melanogaster show higher discrimination than the females of D. simulans.
4. DISCUSSION
The results of this study demonstrate that the females of both Drosophila species (melanogaster and simulans) engaged in assortative mating in response to Wolbachia infection. The females chose male mating partners with their same state of infection, whereas the males mated indiscriminately with either infected or cured females. However the female choice is more evident in D. melanogaster (Table 7).
The different behaviour of both species is not related with the intensity of the CI. Gazla and Carracedo [44] studied these populations and demonstrated that the both

Table 6. D. simulans male choice. Number and comparison of females mating with males in combinations of infected and cured pairs.

Table 7. Comparison between the results of D. melanogaster and D. simulans in female choice tests between infected or cured females.
species have cytoplasmic incompatibility, estimated by the female productivity, and incompatible crosses effect was higher in D. simulans (19.53 ± 3.52) than D. melanogaster (27.93 ± 1.41). This result suggests that the female choice is not related with the fitness loss in incompatible crosses.
Koukou et al. [22] suggest that Wolbachia may alter the pheromones profiles of males or females and affect mating preference, or the bacteria can alter the behaviour in subtle ways that contribute to assortative mating. The authors suggest that the role of Wolbachia in premating isolation could be a side effect of infection, rather than the direct selection of the bacteria to induce mating discrimination.
The different patterns of pheromones or sexual behaviours are caused by differences between genes that affect these characters, however, in this study the infected and cured individuals came from the same population and share the same genetic background, then, the female choice can not be due to genetic differences between infected and uninfected flies. Therefore, the female choice could be due to the presence or absence of the bacteria in flies.
The female’s choice may induce sexual isolation to separate infected individuals from uninfected, acting as a barrier to gene flow between individuals with different status of infection. This sexual isolation combined with the negative effect of CI in incompatible crosses (postmating isolation) can considered the infection of Wolbachia as a source of sympatric speciation [22-25];
In D. melanogaster, Wolbachia can act as an additive factor on sexual isolation in intraspecific level [22]. Moreover these authors detected that the antibiotic treatment used in the elimination of Wolbachia from the infected populations decreased the levels of mating discrimination of infected males and females, but had no effect on the level of mating discrimination between uninfected populations. However, we didn’t detect any effect of antibiotic treatment on mating discrimination, since cured and infected females didn’t show random mating in both species. The differences of the antibiotic effect in sexual discrimination can be due to the different methodology used in our experiment, such as the concentration of tetracycline, the type of mating choice (multiple choices versus female and male choice) and also the flies were cultured three generations without antibiotic before the test starting.
The female’s choice may have several explanations: since the mating between uninfected females with infected males can cause CI, which decreases the female’s fecundity, productivity and general fitness, after a long period of coexistence between infected and uninfected individuals in natural populations, it is possible that natural selection acts against incompatible crosses and increase the incidence of compatible crosses. In these circumstances, it is possible to explain the presence of infected and uninfected flies in nature, and how to maintain this variability.
Since “uninfected” flies used in this experiment are coming from infected strains, and there is not possibility of genetic changes that could have developed or accumulated during the evolution of the population, thus the mating discrimination can not be explained by genetic changes due to natural selection. Similar results were shown in D. melanogaster by Koukou [22], who showed that Wolbachia (and not genetic factors) act as the main contributor on the level of premating isolation between populations.
That infected females prefer to mate with infected males is interesting because CI does not occur in their infected/uninfected crosses. However, crosses between infected pairs have higher rates of egg production and productivity than crosses between uninfected pairs [45], [44], suggesting that Wolbachia are acting as mutualist more than parasite as has been shown by Weeks [45]; this fact could be the responsible of the sexual selection that increases crosses between infected flies and also could explain the spread of the infection in nature and under laboratory conditions.
Understanding how females detect the Wolbachia infection of their possible mate will be an important step to understand the elements that are involved in the mating recognition systems and in the development of sexual isolation. Peng [46] demonstrated that Wolbachia had significant effects on fly behaviour, Wolbachia altered olfactory-cued locomotion in Drosophila simulans, thus increasing their basal activity level as well as their response to food cues. However, in D. melanogaster, Wolbachia caused a slight decrease in the response to food cues, but did not alter the host’s basal activity levels, which suggests that the bacteria could alter different behaviours in the two species.
In conclusion, females from D. melanogaster and D. simulans can detect the presence of Wolbachia, (or other associated undetected bacterium) in the males, and base their choice of mate on this information and giving rise to assortative mating. Wolbachia infection can thus be involved in the process of incipient sympatric speciation, and genetic changes are not necessary for this event. In this sense could be interesting to study the modifications induced by Wolbachia in the sexual behaviours of males.
5. ACKNOWLEDGEMENTS
We would like to thank Professor Pelayo Casares, R. Piñeiro and Amparo Villabrille (Universidad de Oviedo) for technical assistance. This work was supported by the University of Oviedo (Spain) Grant BMC 2001, 2599
REFERENCES
- Dobzhansky, T. (1937) Genetics and the origins of species. Columbia University Press, New York.
- Mayr, E. (1942) Systematic and the origin of species. Columbia University Press, New York.
- Kawanishi, M. and Watanabe, T.K. (1981) Genes controlling courtship song and mating preference in Drosophila melanogaster, D. simulans, and their hybrids. Evolution, 35, 1128-1133. doi:10.2307/2408126
- Paterson, H.E.H. (1985) The recognition concept of species. In Species and speciation. In: Vrba, E.S., Ed., Transvaal Museum Monograph No. 4, Transvaal Museum, Pretoria, 21-29.
- O’Neill, S.L. and Karr, T.L. (1990) Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature, 348, 178-180. doi:10.1038/348178a0
- Bandi, C., Anderson, T.J.C., Genchi, C. and Blaxter, M. (1998) Phylogeny of Wolbachia in filarial nematodes. Proceedings of the Royal Society B: Biological Sciences, 265, 2407-2413. doi:10.1098/rspb.1998.0591
- Werren, J.H. (1997) Biology of Wolbachia. Annual Review of Entomology, 42, 587-609. doi:10.1146/annurev.ento.42.1.587
- Stouthamer, A.J., Breeuwer, A.J., Hurst, G.D.D., et al. (1999) Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annual Review of Microbiology, 53, 71-102. doi:10.1146/annurev.micro.53.1.71
- Jeyaprakash, A. and Hoy, M.A. (2000) Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Molecular Biology, 9, 393-405. doi:10.1046/j.1365-2583.2000.00203.x
- Dobson, S.L., Marsland, E.J. and Rattanadechakul, W. (2002) Mutualistic Wolbachia infection in Aedes albopitus: Accelerating cytoplasmic drive. Genetics, 160, 1087- 1094.
- McGraw, E.A. and O’Neill, S.L. (2003) Wolbachia pipientis: Intracellular infection and pathogenesis in Drosophila. Current Opinion in Microbiology, 7, 67-70. doi:10.1016/j.mib.2003.12.003
- Floate, K.D., Kyei-Poku, G.K. and Coghlin, P.C. (2006) Overview and relevance of Wolbachia bacteria in biocontrol research. Biocontrol Science and Technology, 16, 767-788. doi:10.1080/09583150600699606
- Bourtzis, K., Braig, H.R. and Karr, T.L. (2003) Cytoplasmic Incompatibility. In: Bourtzis, K. and Miller, T., Eds., Insect Symbiosis, CRC Press, Boca Raton, 217-246.
- Hurst, G.D.D., Anbutsu, H., Kutsukake, M. and Fukatsu, T. (2003) Hidden from the host: Spiroplasma bacteria infecting Drosophila do not cause an immune response, but are suppressed by ectopic immune activation. Insect Molecular Biology, 12, 93-97. doi:10.1046/j.1365-2583.2003.00380.x
- Koivisto, R.K.K. and Braig, H.R. (2003) Microorganisms and parthenogenesis. Biological Journal of the Linnean Society, 79, 43-58. doi:10.1046/j.1095-8312.2003.00185.x
- Rigaud, T. (1997) Inherited microorganisms and sex determination of arthropod hosts. In: O’Neill, S.L., Hoffmann, A.A. and Werren, J.H., Eds., Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, Oxford University Press, Oxford, 81-101.
- Werren, J.H. and O’Neill, S.L. (1997) The evolution of heritable symbionts. In: O’Neill, S.L., Hoffmann, A.A. and Werren, J.H., Eds., Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, Oxford University Press, Oxford, 1-41.
- Clancy, D.J. and Hoffmann, A.A. (1998) Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia infected Drosophila simulans. Entomologia Experimentalis et Applicata, 86, 13-24. doi:10.1046/j.1570-7458.1998.00261.x
- Stevens, L. (1989) Environmental factors affecting reproductive incompatibility in flour beetles, genus Tribolium. Journal of Invertebrate Pathology, 53, 78-84. doi:10.1016/0022-2011(89)90076-1
- Kyei-Poku, G.K., Floate, K.D., Benkel, B. and Goettel, M.S. (2003) Elimination of Wolbachia from Urolepis rufipes (Hymenoptera: Pteromalidae) with heat and antibiotic treatments: Implications for host reproduction. Biocontrol Science and Technology, 3, 341-354.
- Aleksandrov, I.D., Aleksandrova, M.V., Goriacheva, I.I., Roshchina, N.V., Shaikevich, E.V. and Zakharov, I.A. (2007) Removing endosymbiotic Wolbachia specifically decreases lifespan of females and competitiveness in a laboratory strain of Drosophila melanogaster. Genetika, 43, 1372-1378.
- Koukou, K., Pavlikaki, H., Kilias, G., Werren, J.H., et al. (2006) Influence of antibiotic treatment and Wolbachia curing on sexual isolation among Drosophila melanogaster cage populations. Evolution: International Journal of Organic Evolution, 60, 87-96.
- Breeuwer, J.A.J. and Werren, J.H. (1990) Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature, 346, 558-560. doi:10.1038/346558a0
- Coyne, J.A. (1992) Genetics and speciation. Nature, 355, 511-515. doi:10.1038/355511a0
- Werren, J.H. and Jaenike, J. (1995) Wolbachia and cytoplasmic incompatibility in mycophagous Drosophila and their relatives. Heredity, 75, 320-326. doi:10.1038/hdy.1995.140
- Vala, F., Egas, M., Breeuwer, J.A. and Sabelis, M.W. (2004) Wolbachia affects oviposition and mating behaviour of its spider mite host. Journal of Evolutionary Biology, 17, 692-700. doi:10.1046/j.1420-9101.2003.00679.x
- Bordenstein, S.H., O Hara, F.P. and Werren, J.H. (2001) Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature, 409, 707-710. doi:10.1038/35055543
- Turelli, M. and Hoffmann, A.A., (1991) Rapid spread of an inherited incompatibility factor in California Drosophila. Nature, 353, 440-442. doi:10.1038/353440a0
- Mercot, H., Llorente, B., Jacques, M., Atlan, A. and Montchamp-Moreau, C. (1995) Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans. Genetics, 141, 1015-1023.
- Hoffmann, A.A., Turelli, M. and Simmons, G.M. (1986) Unidirectional incompatibility between populations of Drosophila simulans. Evolution, 40, 692-701. doi:10.2307/2408456
- Nigro, L. (I991) The effect of heteroplasmy on cytoplasmic incompatibility in transplasmic lines of Drosophila simulans showing a complete replacement of the mitochondrial DNA. Heredity, 66, 41-45. doi:10.1038/hdy.1991.5
- Rousset, F. and Solignac, M. (1995) Evolution of single and double Wolbachia symbioses during speciation in the Drosophila simulans complex. Proceedings of the National Academy of Sciences of the USA, 92, 6389-6393. doi:10.1073/pnas.92.14.6389
- Hoffmann, A.A., Clancy, D.J. and Duncan, J. (1996) Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity, 76, 18. doi:10.1038/hdy.1996.1
- Charlat, S., Bonnavion, P. and Merçot, H. (2003) Wolbachia segregation dynamics and levels of cytoplasmic incompatibility in Drosophila sechellia. Heredity, 90, 157- 161. doi:10.1038/sj.hdy.6800211
- Hoffmann, A.A., Clancy, D.J. and Merton, E. (1994) Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics, 136, 993-999.
- Reynolds, K. and Hoffmann, A.A. (2002) Male age, host effects and the weak expression or non expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genetics Research, 80, 79-87. doi:10.1017/S0016672302005827
- Reynolds, K., Thomson, L.J., Hoffmann, A.A., et al. (2003) The effects of host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcom in Drosophila melanogaster. Genetics, 164, 1027-1034.
- Dean, M.D. (2006) A Wolbachia-associated fitness benefit depends on genetic background in Drosophila simulans. Proceedings of the Royal Society B: Biological Sciences, 273, 1415-1420. doi:10.1098/rspb.2005.3453
- Yamada, R., Floate, K.D., Riegler, M. and O’Neill, S.L. (2007) Male development time influences strength of Wolbachia-induced cytoplasmic incompatibility expression in Drosophila melanogaster. Genetics, 106, 84-86.
- Gomez Valero, L., Soriano Navarro, M., Pérez Brocal, V., Heddi, A., et al. (2004) Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid Cinara cedri. The Journal of Bacteriology, 186, 6626-6633. doi:10.1128/JB.186.19.6626-6633.2004
- Casares, P., Carracedo, M.C., San Miguel, E., Piñeiro, R. and García-Florez, L. (1993) Male mating speed in Drosophila melanogaster: Differences in genetic architecture in relative performance according to female genotype. Behavior Genetics, 23, 349-358. doi:10.1007/BF01067436
- Casares, P., Carracedo, M.C., Del Rio, B., Piñeiro, R., García Flórez, L. and Barros, A.R. (1998) Disentangling the effects of mating propensity and mating choice in Drosophila. Evolution, 52, 126-133. doi:10.2307/2410927
- Prouzan, A. (1976) Effects of age, rearing and mating experiences on frequency dependent sexual selection in Drosophila pseudoobscura. Evolution, 30, 130-145. doi:10.2307/2407680
- Gazla, I.N. and Carracedo, M.C. (2009) Effect of intracellular Wolbachia on interspecific crosses between Drosophila melanogaster and Drosophila simulans. Genetics and Molecular Research, 8, 861-869. doi:10.4238/vol8-3gmr595
- Weeks, AR., Turelli, M., Harcombe, W.R., Reynolds, K.T., et al. (2007) From parasite to mutualist: Rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biology, 5, 997-1005. doi:10.1371/journal.pbio.0050114
- Peng, Y., John, E., Paul, J., et al. (2008) Wolbachia infection alters olfactory-cued locomotion in Drosophila spp. Applied and Environmental Microbiology, 74, 3943-3948. doi:10.1128/AEM.02607-07

