Advances in Chemical Engineering and Science
Vol.05 No.03(2015), Article ID:57575,7 pages
10.4236/aces.2015.53026
Effect of Temperature on the Reaction of 2-(N-acetylamine)-3-(3,5-di-tert-butyl- 4-hydroxyphenyl)-propionic Acid with Oxygen in an Alkaline Condition
A. A. Volodkin1, G. E. Zaikov1, L. N. Kurkovskaja1, S. M. Lomakin1, I. M. Levina1, E. V. Koverzanova2
1Federal State Budgetary Establishment of a Science of Institute of Biochemical Physics of N. M. Emanuelja of Russian Academy of Sciences, Moscow, Russia
2Federal State Budgetary Establishment of a Science of Institute of Chemical Physics of N. N. Semenov of Russian Academy of Sciences, Moscow, Russia
Email: chembio@sky.chph.ras.ru
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 3 March 2015; accepted 27 June 2015; published 30 June 2015
ABSTRACT
Results of oxidation 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid oxygen depend on temperature. At 55˚C - 60˚C, 2,4-di-tert-butylbicyclo(4,3,1)deca-4,6-dien-8-(N-ace- tylamine)-3,9-dion-1-oxa is formed. The constitution is based on dates of spectrums 1Н and 13С NMR. At 95˚C - 97˚C, mixtures of 2,4-di-tert-butylbicyclo(4,3,1)deca-4,6-dien-8-(N-acetylamine)-3,9-dion-1-oxa and of 6,8-di-tert-butyl-3-(N-acetylamine)spiro(4,5)deca-1-oxa-5,8-dien-2,7-dione are produced. Structures are calculated by the method of Hartrii-Foka. Values of enthalpies and of entropies allow to assume dynamic isomerism.
Keywords:
2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic Acid, 6,8-di-tert-butyl-3-(N-acetylamine)spiro(4,5)deca-1-oxa-5,8-dien-2,7-dione Oxidation by Oxygen, 2,4-Di-tert-butylbicyclo(4,3,1)deca-4,6-dien-8-(N-acetylamine)-3,9-dion-1-oxa, NMR-Spectroscopy

1. Introduction
The oxidation by oxygen 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid (analogue tyrosine) is of interest in research of products oxidations in a presence of an antioxidant. Results of early works [1] - [3] have shown possibility to use of a synthesis of analogues tyrosine in research of biology and a investigation of specificity properties in the conditions of a reactions with acid agents. For example, in classical reaction with thionyl chloride instead of acid chloride 2-(N-acetylamine)-2-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid, a oxidative dimerization is produced [4] . There is an eliminate of tert-butyl groups in reaction 2-(N-acety- lamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid with hydrogen chloride [5] .
The direction of oxidation by oxygen 4-replaced 2,6-di-tert-butylphenols depends on conditions and a constitution of substituent and in each specific case results, as a rule, are ambiguous [6] .
In the present work, we studied the oxidation of 2-(N-acetylamine)-2-(3,5-di-tert-butyl-4-hydroxyphenyl)-pro- pionic acid by oxygen. Results of a oxidation of 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-pro- pionic acid may by impotents in process research of products inhibitors of oxidation, especially in the conditions of a biological. We have positioned that in process interaction of 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hy- droxy-phenyl)-propionic acid with oxygen in the presence of alkali are formed 2,4-di-tert-butylbicyclo(4,3,1)- deca-4,6-dien-8-(N-acetylamine)-3,9-dion-1-oxa and 6,8-di-tert-butyl-3-(N-acetylamine)-spiro(4,5)deca-1-oxa- 5,8-dien-2,7-dione. The interrelation of results of oxidation by oxygen depends on temperature.
2. Experimental Part
NMR spectrums registered on the device “Avance-500 Bruker” rather TMS.
2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid it is synthesis on a method [7] , m.p. 204˚C - 206˚C according to [8] : m.p. 204˚C - 206˚C.
Example 1. Oxidation 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid at 55˚C - 60˚C. In solution of 3.35 g (0.01 mol) 2-(N-acetylamino)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid 1.2 g (0.04 mol) NaOH in 60 ml of ethanol at 55˚ - 60˚ passed oxygen within 6 h. In a day to solution added HCl prior to the beginning of breaking (рН ≈ 6). Organic part have separated and after crystallization in the course of solvent evaporation received 0.6 g (≈18%) 2,4-di-tert-butylbicyclo(4,3,1)deca-4,6-dien-8-(N-acetyla- mine)-3,9-dion-1-oxa (2); m.p. 101˚C - 105˚C. Found %: C 68.32 Н 8.34. С19Н27NO4. Calculated %: C 68.44 Н 8.16.
Spectrum 1Н NMR (DMSO-d6, δ, ppm, J/Hz): 1.20 (s, 18Н, tBu); 1.89 (s.3Н, СН3СО); 2.40 (dd, 2Н, СН2СН, J = 12.9,); 4.85 (m. 1Н, СН2СН,); 6.69 (d., 1Н, Ar , J = 2.9); 6.95 (d.,1Н,Ar, J = 2.9); 8.56 (d.,1Н, NH, J = 7.8). Spectrum 13С NMR (DMSO-d6, δ, ppm,): 22.18 (СН3СО); 29.09 (C-CH3); 36.84 (СН2); 48.56 (СН); 76.28 (С); 138.9 (С-H) 139.6 (С-H); 145.3 (С=С) 4 146.0 (С=С) 169.3 (СОNH); 174.0 (COO); 185.4 (C=O).
2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionate sodium (3)
Mixture of 3.35 g (0.01 mol) 1, 0.5 g (0.01 mol) NaOH in 20 ml of ethanol maintain ≈15 min time, solvent evaporate, the residue crystallization from EtOH-H2O (1:1). A yield ≈3 g, m.p. > 250˚. Spectrum 1Н NMR (DMSO-d6, δ, ppm, J/Hz): 1.35 (s., 18 Н, tBu); 1.77 (s., 3Н, СОСН3); 2.73 (dd., 1Н, CH-CHH, J = 6.8); 2.95 (dd., 1Н, CH-CHH, J = 4.6); 3,97 (m. 1Н, СН-СН2); 6.6 (s., 1Н, OH); 6.89 (s. 2Н, Ar); 7.23 (d., 1 Н, J = 7.4). Spectrum 13С NMR (DMSO-d6, δ, ppm): 22.93 (СН3СО); 30.48 (С-СН3); 34.27 (С); 37.45 (СН2); 55.73 (СН); 125.5 (С=С-Н); 130.46 (С-С=С); 138.09 (С-С=С); 151.58 (С-С=О); 167.63 (СОNHCH3); 173.93 (COO).
Example 2. Oxidation 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid at 95˚C - 97˚C. In solution of 3.35 g (0.01 mol) 2-(N-acetylamino)-2-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid, 1.2 g (0.04 mol) NaOH in 60 ml of ethanol at 55˚C - 60˚C passed oxygen within 6 h. Processing and allocation of resultants of reaction made similarly to an example 1. 6,8-Di-tert-butyl-3-(N-acetylamine)spiro(4,5) deca-1- oxa-5,8-dien-2,7-dione from a reaction mixture divide on Al2O3; m.p.102˚C - 105˚C.
Spectrum 1Н NMR (1) (DMSO-d6, δ, ppm, J/Hz): 1.36 (s., 18 Н, tBu); 1.82 (s.3Н, СН3СО); 2.74 (dd. 1Н, CH-CHH, J = 9.2; J = 9.2; J = 13.9); 2.91 (dd.1Н, CH-CHH, J =4.85; J =6.94; J =13.9); 4.32 (m. 1Н, СН-СН2); 6.94 (s, 2Н, Ar); 8.16 (d., 1Н, NH, J = 7.8); Spectrum 1Н ЯМР (2) (DMSO-d6, δ, ppm, J/Hz): 1.36 (s., 18 Н, tBu); 1.80 (s.3Н, СН3СО); 2.74 (d. 2Н, СН2, J =3.2); 4.01 (m. 1Н, СН-СН2); 6.90 (s.2Н, Ar); 8.30 (d., 1Н, NH, J = 7.3);
Spectrum 13С NMR (DMSO-d6, δ, ppm): 22.4 (СН3СО); 26.9 (С); 30.32 (С-СН3); 36.57 (СН2); 53.58 (С1Н); 53.84 (С2Н); 60.18 (C-spiro); 125.07 (С=С1-Н); 125.15 (С=С1-Н); 127.8 (С=С2-Н); 128.4 (С=С2-Н); 145.90 (С1=С); 145.95 (С1=С); 152.24 (С2=C); 152.37 (С2=C); 168.97 (С1ОNH); 169.09 (C2ONH; 171.83 (C1OO); 173.22 (C2OO); 185.37 (C=O).
3. Results and Discussions
In conditions of interaction 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid (1) with NaOH in air atmosphere at ambient temperature 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-pro- pionate sodium is formed, solvable in aqueous-alcoholic solutions. However at temperature of 55˚C - 60˚C oxidation process proceeds, which speed above in oxygen atmosphere. For 6 h reactions »18 % 2,4-di-tert-butyl- bicyclo(4,3,1)deca-4,6-dien-8-(N-acetylamine)-3,9-dion-1-oxa (2) is formed (Scheme 1) At ambient temperature on air salt 3 does not react with oxygen. The composition analysis of reactionary masses and a yield of compound 2 are resulted from the data of the spectrum 1Н NMR (Figure 1).
From the data of the spectrum 1Н NMR follows that signals of 6.69 and 6.95 ppm (J = 2.9 Hz) to belong to two various protons in a hexatomic cycle of structure 2. In the spectrum 13С NMR signals of 138.92 and 139.59 ppm from atoms of carboneum correspond to the data, which analysis in a format debt (Figure 2) specifies in communication of these atoms with hydrogen.
Signal of 185.4 ppm in spectrum 13C NMR (Figure 3) confirms presence of a carbonyl group at a hexatomic cycle of structure 2.

Scheme 1. Structures of compounds 2 and 3.
Figure 1. Spectrum 1H NMR of reactionary mass of reaction compound 1 with oxygen in an alkaline condition at 55˚C - 60˚C for 6 h.
Figure 2. Fragment of the spectrum 13С NMR compound 2 in a format dept.
Figure 3. Spectrum 13C NMR from reactionary mass of compound 1 with oxygen in an alkaline condition at 55˚C - 60˚C for 6 h.
At last, a signal of 76.28 ppm belongs to tetrahedral atom of carboneum, forming communications with cycle carboneums atom, tert-butyl group and atom of oxygen (Figure 4).
According to [9] , a position of a signal from tetrahedral atom of carboneum in spiran to system with atom of nitrogen is in area of 75 ppm.
Data of spectrums NMR is sufficient for conclusions about structure of compound, producing by a interaction of 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid wih O2 at temperature of 50˚C - 60˚C in solution of ethanol. This fact confirms bonding between atom of oxygen of a carboxyl group and of carboneum atom from hexatomic cycle.
In reaction 1 at temperature 95˚C - 97˚C (thermostatic control) 6,8-di-tert-butyl-3-(N-acetylamine)spi-ro(4,5) deca-1-oxa-5,8-dien-2,7-dione (4)\is formed, along with compound 2 (Scheme 2).
The interrelation of resultants of reaction (2 and 4) are found from comparison there of integrals signals in spectrum 1H NMR reactionary mass (Figure 5 and Figure 6).
The analysis of dates of a spectrum specifies in presence at reactionary mass of three compounds are identified: one of them is with structure 2 and two compounds are with structure 4 which, apparently, are structure of isomers (Figure 6).
Signals “doublet of doublets” belong to one of structure 4а, the signal “triplet” belongs to 4b (Scheme 3).
Figure 4. Correlation of data spectrums 1Н and 13С NMR in co-ordinates C-H.

Scheme 2. Structures of compounds isolating in reaction compound 1 with oxygen.
Figure 5. Spectrum 1Н NMR reactionary mass after oxidation of compound 1 by oxygen at 95˚C - 97˚C for 6 h.
Figure 6. Fragment of the spectrum 1Н NMR (2.9 - 2.7 ppm) from a signal from group CH2 of structure 4.
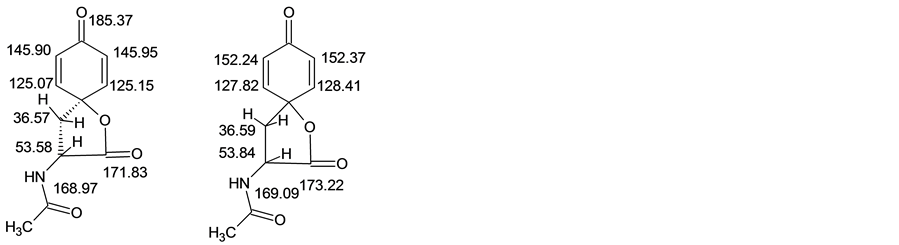
4a 4b
Scheme 3. Isomers of compound 4.
One of the possible factors influencing allocation of frequencies in spectrums 1Н and 13С NMR can be asymmetry because of influence tert-butyl groups on geometrical parameters.
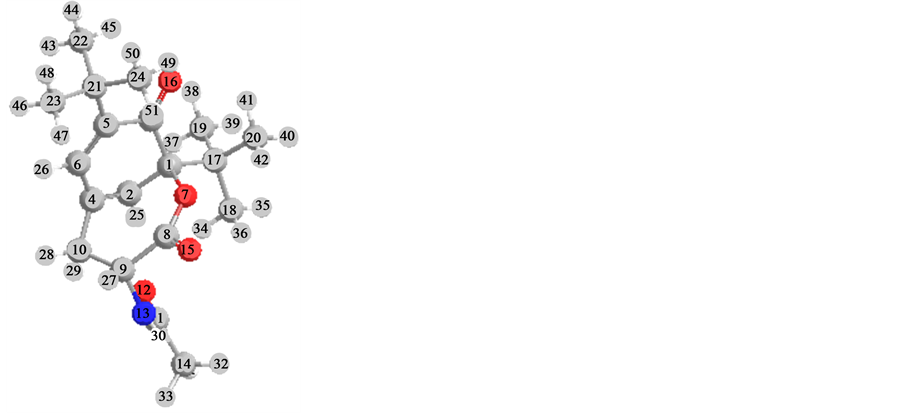
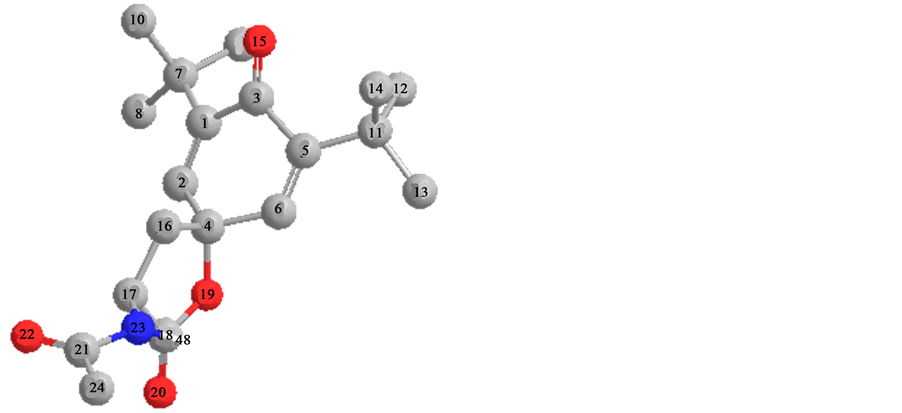
Path comparison of structures 2 and 4 by method of Hartrii-Foka (UHF) on energy formations the result is received: (Hf˚) for 2 Hf˚ = −179.3-for 4 = −144.4 kcal∙mol−1, values of charges on atoms of oxygen [2, O (15) q = −0.58; 4, O (15) q = −0.59].
Distinctions in charges on atoms of carboneum in a hexatomic cycle [2, C (1) q = −0.02, 4, C (1) q = +0.3].
Angle between a plane of hexatomic cycle and communication С=О (15), for 2 [C (2)-C (1)-(O)-(16)] w/ hailstones = 27.6˚, for 4 [C (2) - C (1)-O (15)] w/ hailstones = 26˚.
2 4
A calculates of enthalpies (H˚f) and of entropies (S˚f) for structures 2 and 4 have appeared comparable (2, Hf˚ = 17.5 kcal∙mol−1, Sf˚ = 182.3 unite∙cаl∙К−1∙мол−1; 4, Hfo = 17.1 kcal∙mol−1, Sf˚ = 174.9 unite, cаl∙К−1∙mol−1). These results in of set with calculation of geometry of structures 2 and 4 specifies in dynamic isomerism possibility.
4. Conclusion
At oxidative ring, the formation is produced in process interaction of 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hy- droxyphenyl)-propionic acid with oxygen in the presence of alkali and 2,4-di-tert-butylbicyclo(4,3,1)deca-4,6- dien-8-(N-acetylamine)-3,9-dion-1-oxa and 6,8-di-tert-butyl-3-(N-acetylamine)-spiro(4,5)deca-1-oxa-5,8-di-en- 2,7-dione are formed. The interrelation of results by oxygen depends on temperature. These results may by impotent for investigation of components during reactions 2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)- propionic acid in the conditions of biological researches.
References
- Volodkin, A.A., Erohin, V.N., Burlakova, E.B., Zaikov, G.E. and Lomakin, S.M. (2013) Structure and Biological Properties of Sodium and Potassium 2-(Carboxy)-2-(N-acetylamine)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionates, The Journal of Chemical Physics, 32, 66-72. [In Russian]
- Volodkin, A.A. and Zaikov, G.E. (2014) Mechanism of Myocardial Infarction: Pathological Changes in Rats after Application of Anphen Sodium. Herald of Kazan Technological University, 17, 138-141. [In Russian]
- Volodkin, А.А., Zaikov, G.E., Kurkovskaja, L.N., Evteeva, N.M., Lomakin, S.M., Parshina, E.J., Gendel, L.J. and Rahbanova, Z.M. (2012) Synthesis of Biological Antioxidant in Reaction Etherification 2-(N-acetylamine)-3-(3,5-di- tert-butyl-4-hydroxyphenyl)-propionic Acid. Herald of Kazan Technological University, 15, 177. [In Russian]
- Volodkin, A.A., Kurkovskaja, L.N., Zaikov, G.E. and Lomakin, S.M. (2013) Formation 3,3’,5,5’-Tetra-tert-butyldi- phenoquinone and 3,3’,5,5’-tetra-tert-butyl-4’,4-di-hydroxydi-phenylе in Reaction 2-(N-acetylamine)-3-(3’, 5'-di-tert- butyl-4'-hydroxyphenyl)-propionic Acid with Thionyl Chloride. Herald of Russian Academy of Sciences, Series Chemistry, 2265. [In Russian]
- Volodkin, А.А., Zaikov, G.E., Kurkovskaja, L.N., Lomakin, S.M. and Sofina, S.J. (2013) Synthesis New Antioxidants into Reaction 2-(N-acetylamine)-3-(3’,5’-di-tert-butyl-4’-hydroxy-phenyl)-propionic acid with Hydrogen Chloride. Herald of Kazan Technological University, 16, 18-21. [In Russian]
- Volodkin, A.A., Malysheva, R.D. and Ershov, V.V. (1982) Oxidation Methyl Ether (3,5-di-tert-butyl-4-hydroxy- phenyl)-propionic Acid by Oxygen. Herald of Russian Academy of Sciences, Series Chemistry, 1594. [In Russian]
- Volodkin, AA., Lomakin, S.M., Zaikov, G.E. and Evteeva, N.M. (2009) Alkaline Hydrolysis of Diethyl N-Acetyla- mine-(3,5-di-tert-butyl-4-hydroxybenzyl)malonate. Herald of Russian Academy of Sciences, Series Chemistry, 900. [In Russian]
- Teuder, H.J., Rause, H. and Berariu, V. (1978) 3,5-Di-tert-butyltyrosin. Lieb. Ann, 1978, 757.
- Vystorop, I.V., Konovalova, N.P., Neljubina, J.V., Varfolomeev, V.N., Fedorov, B.S., . Sashenkova, T.E., Berseneva, E.N., Lysenko, K.A. and Kostjanovsky, R.G. (2010) Cycle Hydroxamoving Acids from a-Aminoacids. Herald of Russian Academy of Sciences, Series Chemistry, 127-134. [In Russian]







