Paper Menu >>
Journal Menu >>
 Vol.2, No.9, 1033-1039 (2010) Health doi:10.4236/health.2010.29152 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Cytosolic phospholipase A2 S-nitrosylation in ghrelin protection against detrimental effect of ethanol cytotoxicity on gastric mucin synthesis ——Ghrelin in gastric mucosal protection Bronislaw L. Slomiany, Amalia Slomiany Research Center University of Medicine and Dentistry of New Jersey Newark, Newark, USA; Corresponding Author: slomiabr@umdnj.edu Received 12 March 2010; revised 8 April 2010; accepted 11 April 2010. ABSTRACT Ghrelin, a peptide hormone produced mainly in the stomach, has emerged recently as an im- portant regulator of nitric oxide synthase (NOS) and cyclooxygenase (COX) enzyme systems, the products of which play direct cytoprotective function in the maintenance of gastric mucosal integrity. In this study, using gastric mucosal cells, we report on the role of ghrelin in coun- tering the cytotoxic effect of ethanol on mucin synthesis. We show that the countering effect of ghrelin on mucin synthesis was associated with the increase in NO and PGE2 production, and characterized by a marked up-regulation in cy- tosolic phospholipase A2 (cPLA2) activity. The ghrelin-induced up-regulation in mucin synthe- sis, like that of cPLA2 activity, was subject to suppression by Src inhibitor, PP2 and ERK in- hibitor, PD98059, as well as ascorbate. Moreover, the loss in countering effect of ghrelin on the ethanol cytotoxicity and mucin synthesis was attained with cNOS inhibitor, L-NAME as well as COX-1 inhibitor SC-560. The effect of L-NAME was reflected in the inhibition of ghrelin-induced mucosal cell capacity for NO production, cPLA2 S-nitrosylation and PGE2 generation, whereas COX-1 inhibitor caused only the inhibition in PGE2 generation. Our findings suggest that the activation of gastric mucosal cPLA2 through cNOS-induced S-nitrosylation plays an essen- tial role in the countering effect of ghrelin on the disturbances in gastric mucin synthesis caused by ethanol cytotoxicity. Keywords: Ghrelin; Ethanol Cytotoxicity; Gastric Mucin; CNOS; CPLA2; S-Nitrosylation 1. INTRODUCTION Acute gastric mucosal lesions, mucosal inflammatory changes, and gastro-duodenal ulceration are well-recogni- zed consequences of alcohol abuse on the health of gas- trointestinal tract [1-3]. The gastric mucosal responses to ethanol cytotoxicity are manifested by microcirculatory changes, elevation in proinflammatory cytokine produc- tion, enhancement in epithelial cell apoptosis, and the impairment in prostaglandin and nitric oxide signaling pathways [2,4,5]. Furthermore, the disturbances in mu- cus producing gastric mucosal cells by ethanol affect the synthesis of mucins, the glycoproteins that maintain the integrity and strength of the protective mucus layer which constitutes the pre-epithelial element of gastric mucosal defense [6,7]. Hence, the alterations in the synthesis and processing of gastric mucins induced by ethanol are di- rectly reflected in the impairment of mucus coat function that weakens the inherent resistance of the mucosa to injury, and facilitates the onset of gastric disease [3,6,7]. Recent advances in understanding the nature of fac- tors involved in the maintenance gastric mucosal integ- rity revealed that the extent of mucosal protection against ethanol cytotoxicity is influenced by ghrelin, a 28-amino acid peptide hormone produced mainly in the stomach [4,8,9]. This endogenous ligand for the growth hormone secretagogue receptor, has been implicated in the control of local inflammations, healing experimen- tally induced gastric ulcers, and the protection of gastric mucosa against acute injury by ethanol [4,9,10]. More- over, ghrelin emerged as an important regulator of the cross-talk between NOS and COX enzyme systems [9], the products of which, NO and PGE2, play direct cyto- protective role in maintaining the gastric mucosal integ- rity under normal physiological conditions [11,12]. Indeed, the literature data support the existence of a functional relationship between the products of NOS and  B. L. Slomiany et al. / HEALTH 2 (2010) 1033-1039 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1034 COX systems, and there are strong indications that the enzyme compartmentalization and substrate availability determines the segregated utilization of the respective products in physiological and pathophysiological proc- esses [13-15]. The stimulation of NO production through NOS induction or the exogenous NO donors leads to up- regulation in COX enzymes activation and the increase in prostaglandin generation [14-17], while the inhibition of NOS decreases prostaglandin formation. Moreover, it has been reported that the NO-induced enzyme protein S-nitrosylation impacts the events of cytosolic phos- pholipase (cPLA2) activation, and hence influence the processes associated with arachidonic acid release for prostaglandin synthesis [18]. In our previous report, using rat gastric mucosal cells, we have shown that ghrelin protection against ethanol cytotoxicity involves cNOS-mediated cPLA2 activation for the increase in PGE2 production [19]. Here, we ex- amined the influence of ghrelin on the disturbances in gastric mucin synthesis caused by ethanol. 2. MATERIALS AND METHODS 2.1. Cell Preparation and Mucin Synthesis The gastric mucosal cells, collected from freshly dis- sected rat stomachs, were suspended in five volumes of ice-cold Dulbecco’s modified (Gibco) Eagle’s minimal essential medium (DMEM), supplemented with fungizone (50 µg/ml), penicillin (50 U/ml), streptomycin (50 µg/ml), and 10% fetal calf serum, and gently dispersed by tritu- ration with a syringe, and settled by centrifugation [5]. Following rinsing, the cells were resuspended in the me- dium to a concentration of 2 × 107 cell/ml, transferred in 1 ml aliquots to DMEM in culture dishes containing [3H]glucosamine (110 µCi), used as a marker of mucin synthesis, and incubated under 95% O2-5% CO2 atmos- phere at 37˚C for 16 h [20]. After washing with DMEM containing 5% albumin to remove free radiolabel, the cells were resuspended in fresh DMEM and incubated for 2 h in the presence of 3% ethanol [5]. In the experi- ments evaluating the effect of ghrelin (rat, Sigma), cNOS inhibitor, L-NAME, iNOS inhibitor, 1400 W, Src inhibi- tor, PP2, ERK1/2 inhibitor, PD98059 (Calbiochem), COX-1 inhibitor, SC-560, COX-2 inhibitor, NS398, and ascorbate (Sigma), the cells were first preincubated for 30 min with the indicated dose of the agent or vehicle followed by incubation with ethanol. The viability of cell preparations before and during the experimentation, assessed by Trypan blue dye exclusion assay, was greater than 97%. At the end of the specified incubation period, the cells were centrifuged, washed with phos- phate-buffered saline, and the combined supernatants used for mucin assay. 2.2. Mucin Analysis and Ethanol Cytotoxicity Assay The combined cell wash and incubation medium con- taining 3H-labeled mucin were treated at 4˚C with 10 volumes of 2% phosphotungstic acid in 20% trichloro- acetic acid for 4 h and the formed precipitates were col- lected by centrifugation. The glycoprotein precipitates were dissolved in 6 M urea and chromatographed on a Bio-Gel A-1.5 column, and the mucin fractions eluted in the excluded volume were subjected to analysis for in- corporation of radiolabel and protein content [20]. For the measurement of ethanol-induced cytotoxicity, the aliquots of cell suspension from the control and various experimental conditions were centrifuged at 300 × g for 5 min and the supernatants used for the assay of cyto- toxicity using TOX-7 lactate dehydrogenase assay kit (Sigma). 2.3. PGE2 and NO Quantification The aliquots of cell suspension from the control and various experimental conditions were centrifuged at 1500 × g for 5 min and the conditioned medium super- natant collected. PGE2 assays were carried out using a PGE2 EIA kit (Cayman) and 100 µl aliquots of the spent medium supernatant [5]. To assess nitric oxide produc- tion in rat gastric mucosal cells, we measured the stable NO metabolite, nitrite, accumulation in the culture me- dium using Griess reaction [21]. 2.4. CPLA2 Activity Assay The measurement of cPLA2 activity in the gastric mu- cosal cells following various experimental conditions was carried out using cPLA2 assay kit (Cayman). The cells were homogenized in 1 ml of 50 mM Hepes buffer, pH 7.4, containing 1 mM EDTA, and centrifuged at 10,000 × g for 15 min at 4˚C. The supernatants were filtered through an Amicon YM30 filter concentrators, followed by 15 min incubation with 5 µM of calcium- independent PLA2 inhibitor, bromoenol lactone, and the aliquots (10 µl) of such prepared cell lysates were sub- jected to cPLA2 assay [19]. 2.5. CPLA2 S-Nitrosylation A biotin switch procedure was employed to assess the cPLA2 protein S-nitrosylation [22,23]. The gastric mu- cosal cells, treated with ghrelin (0.8 µg/ml) or L-NAME (300 µM)+ghrelin and incubated for 2 h in the presence of 3% ethanol, were lysed in 0.2 ml of HEN lysis buffer, pH 7.7, and the unnitrosylated thiol groups were blocked with S-methyl methanethiosulfonate reagent [23]. The proteins were precipitated with acetone, resuspended in  B. L. Slomiany et al. / HEALTH 2 (2010) 1033-1039 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1035 0.2 ml of HEN buffer containing 1% SDS, and subjected to targeted nitrothiol group reduction with sodium ascor- bate (100 mM). The free thiols were then labeled with biotin and the biotinylated proteins were recovered on streptavidin beads. The formed streptavidin bead-protein complex was washed with neutralization buffer, and the bound proteins were dissociated from streptavidin beads with 50 µl of elution buffer (20 mM HEPES, 100 mM NaCl, 1 mM EDTA, pH 7.7) containing 1% 2-mercapto- ethanol [22]. The obtained proteins were then analyzed by Western blotting. 2.6. Western Blot Analysis The mucosal cells, collected by centrifugation, were re- suspended for 30 min in ice-cold lysis buffer [19], and following brief sonication the lysates were centrifuged at 12,000 g for 10 min and the supernatants subjected to protein determination using BCA protein assay kit (Pierce). The samples, including those subjected to bio- tin switch procedure, were then resuspended in loading buffer, boiled for 5 min, and subjected to SDS-PAGE using 50 µg protein/lane. The separated proteins were transferred onto nitrocellulose membranes and probed with the antibody (Cell Signaling) against the cPLA2, and after incubation with the horseradish peroxidase- conjugated secondary antibody, the protein bands were revealed using an enhanced chemiluminescence detec- tion. 2.7. Data Analysis All experiments were carried out using duplicate sam- pling, and the results are expressed as means ± SD. Analysis of variance (ANOVA) was used to determine significance and the significance level was set at P < 0.05. 3. RESULTS To assess the influence of ghrelin on the disturbances in gastric mucin synthesis caused by alcohol cytotoxicity, we employed rat gastric mucosal cells exposed to etha- nol at the dose range (3%) that impairs the mucosal ca- pacity for mucin synthesis and prostaglandin generation [1-3]. We determined that preincubation of the mucosal cells with ghrelin led to a concentration-dependent pre- vention of ethanol cytotoxicity, and afforded nearly com- plete protection at 0.8 µg/ml of ghrelin (Figure 1). Fur- ther, our results revealed that cytotoxicity induced in gastric mucosal cells by 3% ethanol was reflected in a 32.6% decrease in mucin synthesis (Figure 1), as well as a 29.8% reduction in PGE2 generation and a 51.7% drop in NO production (Figure 2). Ghrelin at its optimal con- centration (0.8 µg/ml) for the protection against ethanol Figure 1. Effect of ghrelin on ethanol-induced cytotoxicity and the synthesis of mucin in rat gastric mucosal cells. The cells, labeled with [3H]glucosamine as a marker of mucin synthesis, were treated with the indicated concentrations of ghrelin and incubated for 2 h in the presence of 3% ethanol (Et). Values represent the means ± SD of five experiments. *P < 0.05 com- pared with that of control. **P < 0.05 compared with that of Et alone. Figure 2. Effect of ghrelin on ethanol-induced changes in the production of PGE2 and nitrite by gastric mucosal cells. The cells were treated with the indicated concentrations of ghrelin and incubated for 2 h in the presence of 3% ethanol (Et). Val- ues represent the means ± SD of five experiments. *P < 0.05 compared with that of control. **P < 0.05 compared with that of Et alone. cytotoxicity evoked a 55.4% increase in the mucosal cell capacity for mucin synthesis (Figure 1), while NO pro- duction increased 2.1-folds and PGE2 generation by 53.1% (Figure 2). Moreover, we found that significant loss in the pre- ventive effect of ghrelin on the ethanol-induced toxicity and the cell capacity for mucin synthesis was attained with cNOS inhibitor, L-NAME as well as specific COX-1 inhibitor, SC-560, while selective iNOS inhibitor, 1400W and a specific COX-2 inhibitor, NS-398 had no effect (Figure 3). The effect of L-NAME, furthermore, was reflected in the inhibition of ghrelin-induced mucosal cell capacity for NO production as well as PGE2 genera- tion, whereas the pretreatment with COX-1 inhibitor, 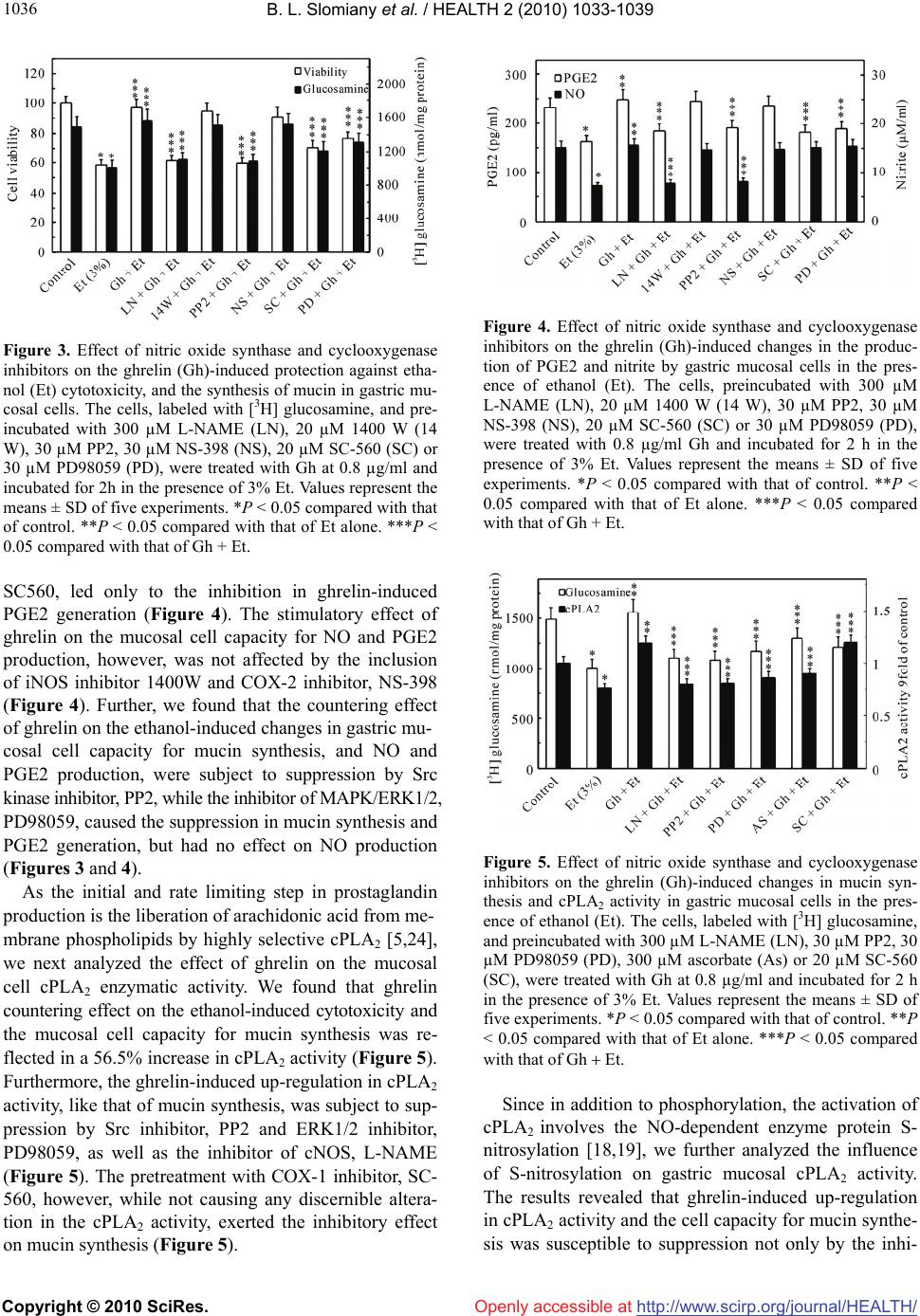 B. L. Slomiany et al. / HEALTH 2 (2010) 1033-1039 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1036 Figure 3. Effect of nitric oxide synthase and cyclooxygenase inhibitors on the ghrelin (Gh)-induced protection against etha- nol (Et) cytotoxicity, and the synthesis of mucin in gastric mu- cosal cells. The cells, labeled with [3H] glucosamine, and pre- incubated with 300 µM L-NAME (LN), 20 µM 1400 W (14 W), 30 µM PP2, 30 µM NS-398 (NS), 20 µM SC-560 (SC) or 30 µM PD98059 (PD), were treated with Gh at 0.8 µg/ml and incubated for 2h in the presence of 3% Et. Values represent the means ± SD of five experiments. *P < 0.05 compared with that of control. **P < 0.05 compared with that of Et alone. ***P < 0.05 compared with that of Gh + Et. SC560, led only to the inhibition in ghrelin-induced PGE2 generation (Figure 4). The stimulatory effect of ghrelin on the mucosal cell capacity for NO and PGE2 production, however, was not affected by the inclusion of iNOS inhibitor 1400W and COX-2 inhibitor, NS-398 (Figure 4). Further, we found that the countering effect of ghrelin on the ethanol-induced changes in gastric mu- cosal cell capacity for mucin synthesis, and NO and PGE2 production, were subject to suppression by Src kinase inhibitor, PP2, while the inhibitor of MAPK/ERK1/2, PD98059, caused the suppression in mucin synthesis and PGE2 generation, but had no effect on NO production (Figures 3 and 4). As the initial and rate limiting step in prostaglandin production is the liberation of arachidonic acid from me- mbrane phospholipids by highly selective cPLA2 [5,24], we next analyzed the effect of ghrelin on the mucosal cell cPLA2 enzymatic activity. We found that ghrelin countering effect on the ethanol-induced cytotoxicity and the mucosal cell capacity for mucin synthesis was re- flected in a 56.5% increase in cPLA2 activity (Figure 5). Furthermore, the ghrelin-induced up-regulation in cPLA2 activity, like that of mucin synthesis, was subject to sup- pression by Src inhibitor, PP2 and ERK1/2 inhibitor, PD98059, as well as the inhibitor of cNOS, L-NAME (Figure 5). The pretreatment with COX-1 inhibitor, SC- 560, however, while not causing any discernible altera- tion in the cPLA2 activity, exerted the inhibitory effect on mucin synthesis (Figure 5). Figure 4. Effect of nitric oxide synthase and cyclooxygenase inhibitors on the ghrelin (Gh)-induced changes in the produc- tion of PGE2 and nitrite by gastric mucosal cells in the pres- ence of ethanol (Et). The cells, preincubated with 300 µM L-NAME (LN), 20 µM 1400 W (14 W), 30 µM PP2, 30 µM NS-398 (NS), 20 µM SC-560 (SC) or 30 µM PD98059 (PD), were treated with 0.8 µg/ml Gh and incubated for 2 h in the presence of 3% Et. Values represent the means ± SD of five experiments. *P < 0.05 compared with that of control. **P < 0.05 compared with that of Et alone. ***P < 0.05 compared with that of Gh + Et. Figure 5. Effect of nitric oxide synthase and cyclooxygenase inhibitors on the ghrelin (Gh)-induced changes in mucin syn- thesis and cPLA2 activity in gastric mucosal cells in the pres- ence of ethanol (Et). The cells, labeled with [3H] glucosamine, and preincubated with 300 µM L-NAME (LN), 30 µM PP2, 30 µM PD98059 (PD), 300 µM ascorbate (As) or 20 µM SC-560 (SC), were treated with Gh at 0.8 µg/ml and incubated for 2 h in the presence of 3% Et. Values represent the means ± SD of five experiments. *P < 0.05 compared with that of control. **P < 0.05 compared with that of Et alone. ***P < 0.05 compared with that of Gh Et. Since in addition to phosphorylation, the activation of cPLA2 involves the NO-dependent enzyme protein S- nitrosylation [18,19], we further analyzed the influence of S-nitrosylation on gastric mucosal cPLA2 activity. The results revealed that ghrelin-induced up-regulation in cPLA2 activity and the cell capacity for mucin synthe- sis was susceptible to suppression not only by the inhi-  B. L. Slomiany et al. / HEALTH 2 (2010) 1033-1039 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1037 bitor of cNOS, L-NAME, but also by ascorbate (Figure 5), which is in keeping with known susceptibility of S-nitrosylated proteins to this reducing agent [14,23]. Moreover, Western blot analysis of the mucosal cell lys- ates subjected to biotin switch procedure, with antibody against cPLA2, revealed that ghrelin prevention of the ethanol-induced cytotoxicity was manifested in the in- crease in cPLA2 protein S-nitrosylation, whereas prein- cubation with L-NAME blocked the ghrelin-induced cPLA2 nitrosylation (Figure 6). 4. DISCUSSION Acute gastric mucosal injury and gastro-duodenal ulcera- tions are the most common consequences of alcohol abuse on the health of gastrointestinal tract [1-3]. More- over, the disturbances in gastric mucosal cells caused by ethanol cytotoxicity affect the synthesis of mucins, the glycoproteins that maintain the integrity and strength of the protective mucus layer, and hence weaken the inher- ent resistance of the mucosa to injury and facilitate the onset of gastric disease [6,7]. Therefore, considering the recent evidence for the involvement of ghrelin in gastric mucosal protection against injury by ethanol [4,9], in the study presented herein we examined the influence of this 28-amino acid peptide hormone on the synthesis of gas- tric mucin. Using rat gastric mucosal cells exposed to ethanol at the concentration range that impairs mucosal cell capac- ity for mucin synthesis and prostaglandin generation [1-3], we demonstrated that the protective effect of ghre- lin against ethanol cytotoxicity was associated with up- regulation in mucin synthesis, and accompanied by the increase in NO and PGE2 production, and the enhance- Figure 6. Effect of cNOS inhibitor, L-NAME (LN) on ghrelin (Gh)-induced cPLA2 S-nitrosylation in gastric mucosal cells exposed to ethanol (Et). The cells were treated with Gh or L-NAME + Gh and incubated for 2 h in the presence of Et. A portion of the cell lysate was processed by biotin switch pro- cedure for protein S-nitrosylation and, along with the reminder of the lysates, subjected to SDS-PAGE, transferred to nitrocel- lulose and probed with anti-cPLA2 antibody. The immunoblots shown are representative of three experiments. ment in cPLA2 activity. A significant loss in the coun- tering effect of ghrelin on the ethanol-induced cytotoxic- ity and the cell capacity for mucin synthesis was attained with cNOS inhibitor, L-NAME as well as specific COX-1 inhibitor, SC-560, whereas COX-2 inhibitor, NS-398, and iNOS inhibitor, 1400W, had no effect. These find- ings are thus in keeping with the results of earlier studies suggesting that the detrimental effect of ethanol on gas- tric mucin synthesis are associated with the impairment in NO and PGE2 generation [1-3,6,25], and lend further credence as to the role of ghrelin in regulation of the cross-talk between NOS and COX enzyme systems [9]. Further, we found that the countering effect of ghrelin on the ethanol-induced changes in mucin synthesis, and NO and PGE2 production, were subject to suppression by Src kinase inhibitor, PP2, whereas the inhibitor of MAPK/ERK, PD98059, elicited suppression in mucin synthesis and PGE2 generation, but had no effect on NO production. Thus the activation of Src appears to be a triggering event whereby ghrelin is capable of affecting the mucosal cell capacity for mucin synthesis as well as NO and PGE2 generation. Moreover, our data on the inhibition of ghrelin-induced mucosal cell capacity for mucin synthesis, and NO and PGE2 generation by L- NAME, and only that of PGE2 and mucin synthesis by COX-1 inhibitor, SC-560, suggest that ghrelin-induced up-regulation in mucin synthesis and PGE2 generation occurs with the involvement of cNOS-derived NO, and requires COX-1 participation. Indeed, the role of NO in modulation of COX enzymes activity in a variety of dif- ferent systems is well documented [15-17]. As the initial and rate limiting event in prostaglandin production is the release of AA from membrane phos- pholipids by highly selective cPLA2 [5,24], we further assessed the influence of ghrelin on the processes of cPLA2 activation. We found that ghrelin elicited up-regu- lation in the mucosal cell cPLA2 activation, which like that of mucin synthesis was subject to suppression by Src inhibitor, PP2, and ERK1/2 inhibitor, PD98059, as well as the inhibitor of cNOS, L-NAME. However, COX-1 inhibitor, SC-560, while not causing discernible alteration in the cPLA2 activity, exerted the inhibitory effect on mucin synthesis. Hence, we concluded that the activation of gastric mucosal cPLA2 by ghrelin for the increase in mucin synthesis to counter ethanol cytotoxic- ity occurs with the involvement of cNOS and requires Src kinase-dependent MAPK/ERK participation. Indeed, the literature data indicate that MAPK/ERK-dependent cPLA2 phosphorylation on the critical Ser505 residue plays a crucial role in Ca2+-dependent translocation of cPLA2 from cytosol to membrane to gain access to AA- rich phospholipid substrates [5,26]. Whilst regulation of the key enzymes for prostaglandin  B. L. Slomiany et al. / HEALTH 2 (2010) 1033-1039 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1038 production through phosphorylation is well recognized, recent evidence indicates that the increase in prostaglan- din formation may also result from NO-induced enzyme protein S-nitrosylation [14,17-19]. Indeed, the post-tran- slational modification through S-nitrosylation at the critical Cys526 residue has been linked to the NO-induced enhancement in catalytic activity of COX-2 [14], and cPLA2 activation through S-nitrosylation at Cys152 was reported to be responsible for up-regulation in arachi- donic acid release in human epithelial cells [18]. There- fore, to assess the role of ghrelin in cPLA2 activation, we further analyzed the influence of S-nitrosylation on gas- tric mucosal cPLA2 activity and the capacity for mucin synthesis. We found that ghrelin-induced up-regulation in cPLA2 activity and the cell capacity for mucin synthe- sis was susceptible to suppression not only by the in- hibitor of cNOS, L-NAME, but also by ascorbic acid, which is keeping with well known susceptibility of S- nitrosylated proteins to this reducing agent [14,23]. Moreover, Western blot analysis of the mucosal cell lys- ates subjected to biotin switch procedure with antibody against cPLA2 revealed that ghrelin prevention of the ethanol-induced cytotoxicity was manifested in the in- creased cPLA2 protein S-nitrosylation, whereas prein- cubation with cNOS inhibitor, L-NAME resulted in the blockage of the ghrelin-induced cPLA2 protein S-nitro- sylation. Therefore, consistent with our results, the cPLA2 activation by ghrelin through S-nitrosylation is of direct relevance to the mucosal capacity for mucin synthesis and PGE2 generation, and hence the protection of gastric mucosal cells against ethanol cytotoxicity. In summary, our study demonstrates that the activa- tion of gastric mucosal cPLA2 through S-nitrosylation plays an essential role in the countering effect of ghrelin on the ethanol-induced disturbances in gastric mucin sy- nthesis. REFERENCES [1] Guslandi, M. (1987) Effects of ethanol on the gastric mucosa. Digestive Diseases Sciences, 5, 21-32. [2] Konturek, S.J., Brzozowski, T., Konturek, W. and Slomiany, B.L. (1993) Growth factors in gastric mucosal integrity, protection and healing of acute and chronic ul- cerations. In: Domschke, W. and Konturek, S.J., Eds., The Stomach, Springer-Verlag, Berlin, 159-176. [3] Slomiany, A., Morita, M., Sano, S., Piotrowski, J., Skro- dzka, D. and Slomiany, B.L. (1997) Effect of ethanol on mucus glycoprotein synthesis, translocation, transport, glycosylation and secretion. Alcoholism: Clinical and Experimental Research, 21, 417-423. [4] Konturek, P.C., Brzozowski, T., Pajdo, R., Ptak, A., Ni- kiforuk, A., Pawlik, W.W. and Hahn, E.G. (2004) Ghrelin -A new gastroprotective factor in gastric mucosa. Journal of Physiology and Pharmacology, 55(2), 325- 336. [5] Slomiany, B.L. and Slomiany, A. (2008) Src kinase- mediated parallel activation of prostaglandin and consti- tutive nitric oxide synthase pathways in leptin protection of gastric mucosa against ethanol cytotoxicity. Journal of Physiology and Pharmacology, 59(2), 301-314. [6] Slomiany, B.L. and Slomiany, A. (1993) Mucus and gas- tric mucosal protection. In: Domschke, W. and Konturek, S.J., Eds., The Stomach, Springer-Verlag, Berlin, 116- 143. [7] Silen, W. (1987) Gastric mucosal defense and repair. In: Johnson, L.R., Ed., Physiology of the Gastrointestinal Tract, 2nd Edition. Raven Press, New York, 1055-1069. [8] Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H. and Kangawa, K. (1999) Ghrelin is a growth-hormone- releasing acylated peptide from stomach. Nature, 402 (6762), 656-660. [9] Sibilia, V., Pagani, F., Rindi, G., Lattauda, N., Rapetti, D., De Luca, V., Campanini, N., Bulgarelli, I., Locatelli, V., Guidobono, F. and Netti, C. (2008) Central ghrelin gas- troprotection involves nitric oxide/prostaglandin cross-talk. British Journal of Pharmacology, 154(3), 688-697. [10] Ceranowicz, P., Warzecha, Z., Dembinski, A., Sendur, R., Cieszkowski, J., Ceranowicz, D., Pawlik, W.W., Kuwa- hara, A., Kato, I. and Konturek, P.C. (2009) Treatment with ghrelin accelerates the healing of acetic acid-in- duced gastric and duodenal ulcers in rats. Journal of Physiology and Pharmacology, 60(1), 87-98. [11] Whittle, B.J., Lopez-Belmonte, J. and Moncada, S. (1990) Regulation of gastric mucosal integrity by endogenous nitric oxide: Interactions with prostanoids and sensory neuropeptides in the rat. British Journal of Pharmacol- ogy, 99(3), 607-611. [12] Peskar, B.M. (2001) Role of cyclooxygenase isoforms in gastric mucosal defense. Journal of Physiology (Paris) 95(1-6), 3-9. [13] Price, K. and Hanson, P. (1998) Constitutive nitric oxide synthase in rat gastric mucosa: Subcellular distribution, relative activity and different carboxyl-terminal anti- genicity of the neuronal form compared with cerebellum. Digestion, 59, 308-313. [14] Kim, S.F., Huri, D.A. and Snyder, S.H. (2005) Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science, 310(5756), 1966-1970. [15] Clancy, R., Varenika, B., Huang, W., Ballou, L., Attur, M., Amin, A.R. and Abramson, S.B. (2000) Nitric oxide synthase/COX cross-talk: Nitric oxide activates COX-1 but inhibits COX-2 derived prostaglandin production. Journal of Immunology, 165(3), 1582-1587. [16] Marnett, L.J., Wright, T.L., Crews, B.C., Tannenbaum, S.R. and Morrow, J.D. (2000) Regulation of prostaglan- din biosynthesis by nitric oxide is revealed by target dele- tion of inducible nitric-oxide synthase. Journal of Bio- logical Chemistry, 275(18), 13427-13430. [17] Cuzzocrea, S. and Salvemini, D. (2007) Molecular me- chanisms involved in the reciprocal regulation of cyclo- oxygenase and nitric oxide synthase enzymes. Kidney International, 71(4), 290-297. [18] Xu, L., Han, C., Lim, K. and Wu, T. (2008). Activation of cytosolic phospholipase A2 through nitric oxide-induced S-nitrosylation. Involvement of inducible nitric-oxide syn- thase and cyclooxygenase-2. Journal of Biological Chem-  B. L. Slomiany et al. / HEALTH 2 (2010) 1033-1039 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1039 istry, 283(6), 3077-3087. [19] Slomiany, B.L. and Slomiany, A. (2009) Involvement of constitutive nitric oxide synthase in ghrelin-induced cy- tosolic phospholipase A2 activation in gastric mucosal cell protection against ethanol cytotoxicity. Inflammo- pharmacology, 17(6 ), 245-253. [20] Slomiany, B.L. and Slomiany, A. (2004) Platelet-activa- ting factor mediates H. pylori lipopolysaccharide inter- ference with gastric mucin synthesis. IUBMB Life, 56(1), 41-46. [21] Wagner, D.A., Glogowski, J., Skipper, P.L., Wishnok, J.S. and Tannenbaum, S.R. (1982) Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Journal of Analyti- cal Biochemistry, 126(1), 131-138. [22] Jaffrey, S.R., Erdjument-Bromage, H., Ferris, D., Tempst, P. and Snyder, S.H. (2001) Protein S-nitrosylation: A physiological signal for neuronal nitric acid. Nature Cell Biology, 3(2), 193-197. [23] Forrester, M.T., Forrester, M.W. and Stamler, J.S. (2007) Assessment and application of the biotin switch tech- nique for examining protein S-nitrosylation under condi- tions of pharmacologically induced oxidative stress. Journal of Biological Chemistry, 282(19), 13977-13983. [24] Sapirstein, A. and Bonventre, J.V. (2000) Specific roles of phospholipase A2 as defined by gene knockout. Bio- chimica et Biophysica Acta, 1488(1-2), 139-148. [25] Slomiany, B.L. and Slomiany, A. (2002) Nitric oxide as a modulator of gastric mucin synthesis: Role of ERK and p38 MAPK activation. IUBMB Life, 54(5), 267-273. [26] Hirabayashi, T. and Shimizu, T. (2000) Localization and regulation of cytosolic phospholipase A2. Biochimica et Biophysica Acta, 1488(1-2), 124-138. |

