Open Journal of Synthesis Theory and Applications
Vol.3 No.3(2014), Article
ID:48317,10
pages
DOI:10.4236/ojsta.2014.33005
Limiting the Migration of Bisphenol A from Polycarbonate Using Dielectric Barrier Discharge
Emad A. Soliman1, Ahmed Samir2*, Ali M. A. Hassan3, Mohamed S. Mohy-Eldin1, Gamal Abd El-Naim1
1Department of Polymer Materials Research, Advanced Technology and New Materials Research Institute, SRTA-City, New Bourg El-Arab City, Alexandria, Egypt
2Center of Plasma Technology, Al-Azhar University, Nasr City, Cairo, Egypt
3Department of Chemistry, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt
Email: *ahmed_samir_aly@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 14 May 2014; revised 18 June 2014; accepted 3 July 2014
ABSTRACT
Dielectric barrier discharge is used as a cheap technique for surface treatment of polycarbonate. The discharge system is working in open air at atmospheric pressure. The treatments are carried out at low discharge powers (1.5 and 2 W) for treatment time (2.5 - 15 min). The treated samples show decrease in the contact angle and increase in the crystallinity, thermal stability and surface roughness. The effect of ozone on the increase in the oxygen containing functional groups is discussed. The treatment process shows effective limitation of the migration of bisphenol A from the surface of polycarbonate due to the cross linking. Zero migration of bisphenole A is recorded as the sample is treated for 7.5 min. The treatment process is found to be very efficient with very low cost.
Keywords:Dielectric Barrier Discharge, Polycarbonate, Bisphenole A, Surface Treatment

1. Introduction
Bisphenol A, (2, 2-bis (4-hydroxyphenyl) propane, (BPA)) is a chemical used primarily as a monomer in the production of polycarbonate plastic (PC). Polycarbonate (PC) is widely used, especially in developing countries, in food contact materials such as infant feeding bottles, tableware (plates, mugs, jugs, and beakers), microwave ovenware, food containers, water bottles, milk and beverage bottles, processing equipment and water pipes. BPA can migrate into food from food containers made of polycarbonate plastic. The human BPA exposure from different sources has been proved [1] [2] . Later BPA has been discovered to be a carcinogen and cause many other health problems [3] -[7] . There are many recent studies that confirm the migration of BPA, especially from baby bottles made of polycarbonate, with dangerous rates affecting the health of infants [8] -[12] .
Plasma has been introduced as an effective technique for treatment of polycarbonate surface. Different properties of the surface of polycarbonate can be changed by plasma treatment [13] -[17] . One of the important advantages of plasma treatment is that: it changes the surface properties of the polycarbonate without altering the bulk properties. The economical impact prevents the wide spread of plasma treatments of surfaces in commercial applications. Usually low pressure plasma systems are very expensive where a vacuum system is needed. Also the power supplies raise the price of plasma system, especially when using RF or microwave power supplies. In addition, the running cost of the treatment process including gases and electric power consumption is another charge that makes the plasma treatment relatively an expensive treatment technique.
In the present work a dielectric barrier discharge (DBD) system, working in open air at atmospheric pressure, is introduced as a cheap source of plasma that overcomes the economical disadvantages of plasma treatment systems. For the first time, DBD has been used in the treatment of polycarbonate surface for the sake of limiting the migration of BPA. The effect of DBD treatment on the surface properties of polycarbonate samples has been studied.
2. Experimental Setup
The experimental arrangement of DBD plasma reactor used in the present treatment is shown in figure 1. The DBD cell consisted of two electrodes of stainless steel discs, and each one has a diameter of 20 cm and thickness of 2 mm. The lower and upper electrodes were fixed to a perspex base of 40 cm diameter and 2 cm thickness. A dielectric material made of glass has a thickness of 1.2 mm was pasted. The upper and lower perspex discs were collected to each other via rubber O-ring. The gap distance between the dielectric glass and the lower electrode was 3 mm. The cell was fed by air via the gas inlet where the air filled the gap space and then exhausted through the gas outlet. Before any treatments the gas is left to flow in the cell for about 5 minutes to sweep any impurities in the reactor. The gas pressure was kept at atmospheric pressure and the gas flow rate is 0.1 L/min. The two electrodes were connected to a high voltage AC power supply of 50 Hz frequency and a variable voltage of 0 - 10 kV. A limiting resistance of 250 kΩ was used to limit the discharge current. The applied voltage was measured via a resistive potential divider (PD) 500:1. The discharge current was measured by measuring the potential drop across a resistance of 1 kΩ. The accumulated charge on the electrodes was measured by measuring the potential drop across a capacitor of 14 μF. The waveforms of the voltage, current and charge was measured by a digital storage oscilloscope (Model HM-407). The concentration of ozone formed inside the DBD system was measured using ozone analyzer (model AFX H1). The exhaust gas of the DBD cell was collected by the analyzer
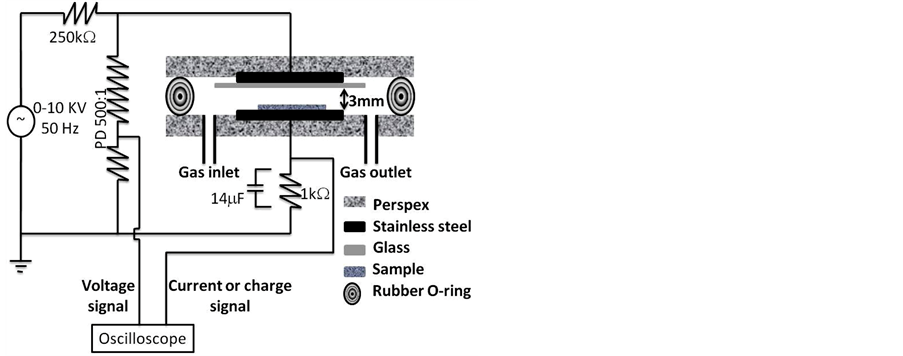
Figure 1. Schematic diagram of DBD plasma reactor.
and measured with accuracy less than 0.1 g/m3.
PC samples were taken from baby bottles which were collected from the local market in Egypt. The samples were cut into 3 × 2 cm2. The treated samples were fixed at the lower electrode where the upper surface of the sample was exposed to the plasma reactive species. For double face treatments, the samples were retreated on the other surface at the same treatment conditions. The samples were treated in the DBD system at different treatment times (2.5 - 15 min) and different discharge currents (1 and 1.5 mA).
The properties of the samples were examined using different techniques. X-Ray Diffraction (XRD) patterns of the samples were recorded by Shimadzu-XRD-7000 Diffractometer—Japan, operating at room temperature with Cu (Kα) radiations of wavelength (λ = 1.5406 Å), generated at 30 Kv - 30 mA. The 2θ range for all samples was in the range from 4˚ to 80˚ with scan speed 0.2˚/s.
Thermo-Gravimetric Analysis (TGA) (Model TGA-50H, Shimadzu—Japan) was used to study the changes in thermal stability of the treated samples. The measurements were carried out with a heating rate of 10˚C/min under flow of N2 gas where the temperature were elevated up to 600˚C.
The contact angle measurements were carried out using the sessile drop method. A contact angle goniometer (ramé-hatr Model 500) using an optical subsystem was used to capture the profile of a pure drop of water on the surface of the sample and then the contact angle was measured.
Scanning Electron Microscope (SEM) was used to study the morphology of the surface of the samples. The samples were coated with gold by the use of Carbon Sputter Coater SPI, Module control, SPI supplies—USA. After coating the samples were placed in the cavity of JEOL JSM-6390 LA, Scanning Electron Microscope (SEM), with a power supply of 30 kV was used as an electron source, and the magnification was X = 7500.
Gas Chromatography/Mass spectrometry GC/MS (Model GC-2010 with GC/MS-QP 2010 Shimadzu—Japan) was used in the present work to measure the migration of BPA from the polycarbonate samples. The samples were put in a beaker with the stimulant (Methy-tert-butyl ether, from Fisher Sci., UK, HPLC Grade), then shacked at a water bath shaker for one hour, then collected at a glass vials to the chromatographic analysis. GC-MS is frequently used for the determination of BPA concentration because of its high accuracy [18] -[21] .
3. Results and Discussion
Figure 2 shows the voltage and current waveforms of the DBD. The onset voltage of the discharge in the present conditions has been found to be 3.5 kV. As the discharge is set in the gap space the discharge current flow as filamentary current, see figure 2. The appearance of the filamentary current in DBD is attributed to the fact that: in the case of DBD, plasma is formed in narrow channels (with diameter of few tens to few hundreds of nm) with short duration time (few tens of ns), these channels are called micro-discharge filaments and the discharge in this case is called filamentary discharge [22] [23] . The filamentary discharge is formed according to the following mechanism. As the applied voltage between the two electrodes exceeds the onset voltage, the free electrons in the gap space are accelerated by the electric field to energies that equal or exceed the ionization energy of the gas, and hence create an avalanche in which the number of electrons doubles with each generation of ionizing collision. The high mobility of the electrons compared to the ions allows the electron swarm to move across the gap in nanoseconds. The electrons leave behind the slower ions, and various excited and active species that may undergo further chemical and physical reactions with the treated samples in the discharge gap. When the electron swarm reaches the opposite electrode, the electrons spread out over the dielectric layer, counteracting the positive charge on the instantaneous anode. This factor combined with the cloud of slower ions left behind reduces the electric field in the vicinity of the filament and terminates any farther ionization along the original track in time scales of tens of nanoseconds [24] . So, micro-discharge filaments are generated individually in the discharge gap. The phase difference between current and voltage, shown in figure 2, indicates to the capacitive reactance of the DBD cell.
According to the design of present treatment system, the running cost of the treatment process depends only on the consumed power in the discharge cell. To evaluate the consumed power in the DBD cell, it is wrong to express it as the product of current and voltage because of the capacitive reactance of the cell and the filamentary behavior of the current. In the present work, the consumed power has been measured using Lissajous method [25] where the voltage difference between the two electrodes has been measured as a function of the charge on the electrodes (Lissajous curve). Figure 3 shows both the discharge current and the consumed power in the
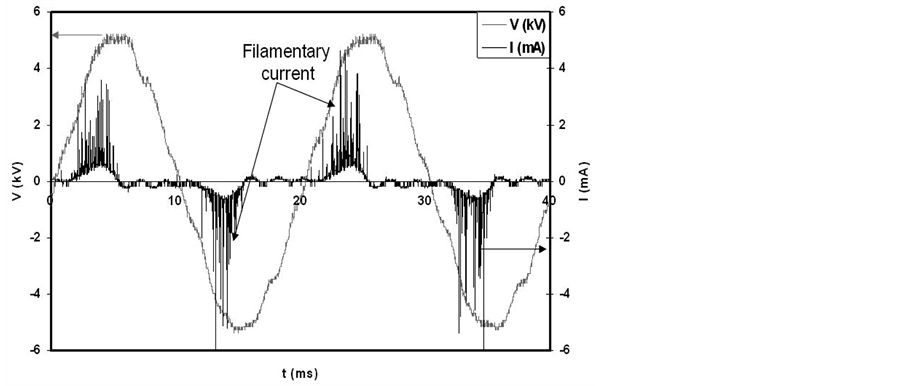
Figure 2. Voltage and current waveforms of DBD.

Figure 3. Discharge current and consumed power in the DBD cell as a function of the discharge voltage.
DBD cell as a function of the discharge voltage. The consumed power in the DBD cell has been found to be very low, even at high voltage (around 8 kV) the consumed power is less than 8 W. In the present work the treatment processes has been carried out at two applied voltages 4 and 5 kV which correspond to discharge currents 1 and 1.5 mA and consumed powers 1.5 and 2 W respectively. The gas temperature under these conditions varies from 30˚C to 40˚C. At higher discharge voltages the gas temperature is elevated to unwanted values which decrease the efficiency of the treatment process.
Figure 4 shows the XRD spectra of the untreated and treated PC samples. The two spectra are characterized by the appearance of halos extending in 2θ range from 15˚ to 18˚. The ratio of diffraction peak areas was used for the analysis of crystal structure. The profile of the halos shows that the polymer is a partly crystalline polymer with a dominant amorphous phase. The peak has been found to be little more intense for the plasma treated sample which indicates to a little increase in crystallinity. There are no remarkable changes in the shape or position of the diffraction peaks after plasma treatment.
The decomposition behavior and thermal stability of the untreated and plasma treated PC samples have been studied using TGA. The thermograms of the untreated and the treated samples are shown in figure 5. The

Figure 4. XRD spectra of the untreated and treated (1.5 W & 7.5 min) PC samples.

Figure 5. TGA thermograms of the untreated and the treated (1.5 W & 7.5 min) PC samples.
weight loss of the untreated PC sample starts at 400˚C, while it starts at 440˚C for the plasma treated sample. The weight loss reaches a maximum at 570˚C with a value of around 76% for the untreated sample and reaches a maximum at 590˚C with a value of around 70% for the plasma treated samples. These results show that the thermal stability of the PC samples has been increased due to the plasma treatment.
The weight loss of PC sample in TGA is attributed to the decomposition (thermal and oxidative) of carbonate link in between the monomers of BPA with vaporization and elimination of volatile products [26] . The increase in the resistance of PC by plasma treatment reveals that the cross-linking dominants or formation of more organized structure seems to be happening as due to the plasma treatment.
Figure 6 shows the contact angle between a drop of distilled water (around 2 μL in volume) and the surface of PC samples (untreated and treated at different treatment times and powers). The measurements of the contact angle have been carried out 7 days after the treatment by DBD. It can be noticed that the contact angle decreases

Figure 6. Contact angle as a function of the treatment time at two different discharge powers.
to around its half value by plasma treatment showing a saturation behavior with both the treatment time (from 2.5 to 15 min) and the discharge power (1.5 and 2 W). The decrease in contact angle can be attributed to increase in surface roughness and incorporation of hydrophilic functional groups which increases the surface energy and hence decreases the contact angle.
The increase in surface roughness has been proved by the SEM examination of untreated and treated samples, shown in figure 7. Such increase in the surface roughness of the treated sample can be referred to the etching process of PC surface by plasma active species (electrons, ions and UV). In the beginning, the etching process is carried out by removal of low-molecular contaminates such as additive and absorbed species. After that the etching process starts to ablate the polymer chain itself. These etching processes are due to the physical removal of molecules by the impact of energetic plasma species and by the breaking up of bonds and chain scission [27] .
The incorporation of hydrophilic functional groups is mainly related to the increase in the concentration of oxygen containing functional groups at the PC surface [28] . In low pressure plasma treatments, the increase in the concentration of oxygen containing functional groups was referred to the oxidation of the polymer surface by atomic oxygen generated by plasma dissociation of oxygen molecules [29] . In the present work we are attributing the oxidation process of PC surface not only to atomic oxygen but also to ozone molecules. Inside the micro-discharge filaments the energetic electrons react with the oxygen molecules forming excited atomic oxygen [30] :
 (1)
(1)
At atmospheric pressure there is a high probability of the reaction between the excited atomic oxygen and the oxygen molecules to form ozone molecules [31] :
 (2)
(2)
The formation of ozone molecules has been studied as a function of the discharge power by measuring the ozone concentration in the exhaust gas of the DBD cell using ozone analyzer (model AFX H1). This relation is shown in figure 8. The increase of the ozone concentration with the discharge power can be attributed to the increase in the electron density and electron energies which increase the reactions of ozone formation (reaction 1 and 2). The saturation effect at discharge powers higher than 4 W can be referred to the elevation of the gas temperature which increases the dissociation rate of ozone molecules. At discharge powers (1.5 and 2 W) valuable concentrations of ozone (2 and 3 g/m3 respectively) have been measured. The oxidation potential of ozone (2.1 V) is comparable to that of atomic oxygen (2.4 V) [32] . These results support our claim about the important role of ozone in the oxidation process of PC surface.
Figure 9 shows the density of BPA migrated from untreated and treated PC samples at different discharge powers and treatment time. The migration density of BPA from the untreated PC sample is around 5.5 ppm. The migration decreases for both discharge powers with treatment time up to 7.5 min and increases again at treat-
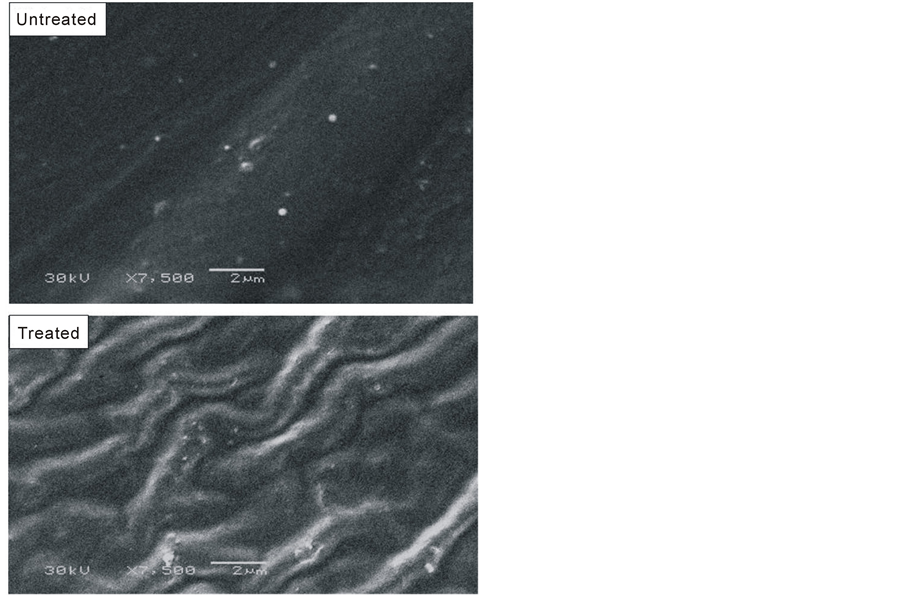
Figure 7. SEM micrographs of the untreated and treated (1.5 W & 7.5 min) PC samples.

Figure 8. Ozone concentration in the exhaust gas of the DBD cell as a function of the discharge power.
ment time of 10 min. The limitation of BPA migration by plasma treatment up to 7.5 min can be referred to the effect of surface treatment of PC samples by plasma species. Plasma species break chemical bonds leaving free radicals at the PC surface. Ozone molecules and atomic oxygen react with the free radicals to produce new functional groups. The new fictional groups increase the cross-linking at the PC surface and hence increase the resistance to the dissociation of BPA monomers. The increase in the resistance to the dissociation by the treatment process has been indicated in TGA measurements. The cross linking at the surface of PC can explain the increase in the crystallinity of the treated samples showed by XRD pattern.
The increase in BPA migration at 10 min can be explained by the effect of etching process. At long treatment

Figure 9. The migration of BPA from PC samples as a function of the treatment time at different consumed powers.
Table 1. Migration of BPA and the cost of the treatment process expressed as the surface density of consumed energy at different treatment conditions.
time (10 min) the temperature of a thin layer at the surface of the sample is elevated due to the impact of the plasma species on the sample surface and due to the elevation of the gas temperature. Such elevation of the surface temperature of the sample increases the etching rate whereas the etching of the treated layer is faster than the treatment of new layer. So, keeping the sample in low temperature is very important for efficient treatments.
As discussed above the most important advantage of the present system is the low cost of the treatment process. The cost of the treatment process here depends only on the consumed electric energy. The surface density of consumed energy, i.e., the consumed electric energy per unit area of the treated PC samples, has been calculated and shown in table 1. It can be notice that: at optimum treatment conditions, discharge power 1.5 W and treatment time of 7.5 min, the surface density of the consumed energy is only about 0.009 kWh/m2 which is very low cost compared with the cost of the syntheses of PC. According to the present work, DBD is recommended as an economical treatment technique of PC instead of using more expensive materials or more expensive treatment techniques.
4. Conclusion
Dielectric barrier discharge has been found to be a cheap and effective technique for surface treatment of polycarbonate. The system has shown effective treatment of PC surface even at low consumed power and short treatment time. Examinations of the untreated and treated samples have shown decrease in the contact angle and increase in the crystallinity, thermal stability, and surface roughness. Effect of etching processes on the surface roughness has been indicated. The effect of ozone on the increase in the oxygen containing functional groups has been discussed. Effect of the treatment process on the migration of BPA from the surface of PC has been studied. Optimum limitation of the migration of BPA has been found at treatment time of 7.5 min where there is no any migration of BPA recorded. At longer time of treatment the migration of BPA increases again where the ablation of the treated layers due to high etching rate becomes more effective than the treatment process. The treatment process has been found to be very efficient in the limitation of the migration of BPA with very low cost.
References
- Vandenberg, L.N., Hauser, R., Marcus, M., Olea, N. and Welshons, W.V. (2007) Human Exposure to Bisphenol A (BPA). Reproductive Toxicology, 24, 139-177. http://dx.doi.org/10.1016/j.reprotox.2007.07.010
- Biles, J.E., McNeal, T.P., Begley, T.H. and Hollifield, H.C. (1997) Determination of Bisphenol-A in Reusable Polycarbonate Food-Contact Plastics and Migration to Food-Simulating Liquids. Journal of Agricultural and Food Chemistry, 43, 3541-3544. http://dx.doi.org/10.1021/jf970072i
- Swan, S.H. (2000) Intrauterine Exposure to Diethylstilbestrol: Long-Term Effects in Humans. APMIS, 108, 793-804. http://dx.doi.org/10.1111/j.1600-0463.2000.tb00001.x
- Klip, H., Verloop, van Gool, J.D., Koster, M.E., Burger C.W. and van Leeuwin, F.E. (2002) Hypospadias in Sons of Women Exposed to Diethylstilbestrol in Utero: A Cohort Study. The Lancet, 359, 1102-1107. http://dx.doi.org/10.1016/S0140-6736(02)08152-7
- Troisi, R., Hatch, E.E., Titus-Ernstoff, L., Hyer, M., Palmer, J.R., Robboy, S.J., Strohsnitter, W.C., Kaufman, R., Herbst, A.L. and Hoover, R.N. (2007) Cancer Risk in Women Prenatally Exposed to Diethylstilbestro. International Journal of Cancer, 121, 356-360. http://dx.doi.org/10.1002/ijc.22631
- Li, D., Zhou, Z., Qing, D., He, Y., Wu, T., Miao, M., Wang, J., Weng, X., Ferber, J.R., Herrinton, L.J., Zhu, Q., Gao, E., Checkoway, H. and Yuan, W. (2010) Occupational Exposure to Bisphenol-A (BPA) and the Risk of Self-Reported Male Sexual Dysfunction. Human Reproduction, 25, 519-527. http://dx.doi.org/10.1093/humrep/dep381
- Kubwabo, C., Kosarac, I., Stewart, B., Gauthier, B.R., Lalonde, K. and Lalonde, P.J. (2009) Migration of Bisphenol A from Plastic Baby Bottles, Baby Bottle Liners and Reusable Polycarbonate Drinking Bottles. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 26, 928-937. http://dx.doi.org/10.1080/02652030802706725
- Cao, X.-L. and Corriveau, J. (2008) Migration of Bisphenol A from Polycarbonate Baby and Water Bottles into Water under Severe Conditions. Journal of Agricultural and Food Chemistry, 56, 6378-6381. http://dx.doi.org/10.1021/jf800870b
- De Coensel, N., David, F. and Sandra, P. (2009) Study on the Migration of Bisphenol-A from Baby Bottles by Stir Bar Sorptive Extraction-Thermal Desorption-Capillary GC-MS. Journal of Separation Science, 32, 3829-3836. http://dx.doi.org/10.1002/jssc.200900349
- Bredey, C., Fjeldalz, P., Skjevraky, I. and Herikstady, H. (2003) Increased Migration Levels of Bisphenol A from Polycarbonate Baby Bottles after Dishwashing, Boiling and Brushing. Food Additives and Contaminants, 20, 684-689. http://dx.doi.org/10.1080/0265203031000119061
- Nam, S.-H., Seo, Y.-M. and Kim, M.-G. (2010) Bisphenol A Migration from Polycarbonate Baby Bottle with Repeated Use. Chemosphere, 79, 949-952. http://dx.doi.org/10.1016/j.chemosphere.2010.02.049
- Palmer, J.R., Wise, L.A., Hatch, E.E., Troisi, R., Titus-Ernstoff, L., Strohsnitter, W., Kaufman, R., Herbst, A.L., Noller, K.L., Hyer, M. and Hoover, R.N. (2006) Prenatal Diethylstilbestrol Exposure and Risk of Breast Cancer. Cancer Epidemiology, Biomarkers Prevention, 15, 1509. http://dx.doi.org/10.1158/1055-9965.EPI-06-0109
- Hofrichter, A., Bulkin P. and Drevillon, B. (2002) Plasma Treatment of Polycarbonate for Improved Adhesion. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 20, 245. http://dx.doi.org/10.1116/1.1430425
- Kitova, S., Minchev, M. and Danev, G. (2005) RF Plasma Treatment of Polycarbonate Substrates. Journal of Optoelectronics and Advanced Materials, 7, 2607-2612.
- Subedi, D.P., Madhup, D.K., Adhikari, K. and Joshi, U.M. (2008) Plasma Treatment at Low Pressure for the Enhancement of Wettability of Polycarbonate. Indian Journal of Pure & Applied Physics, 46, 540-544.
- Qureshi, A., Shah, S., Pelagade, S., Singh, N.L., Mukherjee, S., Tripathi, A., Despande, U.P. and Shripathi, T. (2010) Surface Modification of Polycarbonate by Plasma Treatment. Journal of Physics: Conference Series, 208, Article ID: 012108.
- Vijayalakshmi, K.A., Mekala, M., Yoganand, C.P. and Navaneetha Pandiyaraj, K. (2011) Studies on Modification of Surface Properties in Polycarbonate (PC) Film Induced by DC Glow Discharge Plasma. International Journal of Polymer Science, 2011, Article ID: 426057. http://dx.doi.org/10.1155/2011/426057
- Biles, J.E., McNeal, T.P. and Begley, T.H. (1997) Determination of Bisphenol-A in Reusable Polycarbonate Food-Contact Plastics and Migration to Food-Simulating Liquids. Journal of Agricultural and Food Chemistry, 45, 3541. http://dx.doi.org/10.1021/jf970072i
- Gonzalez-Casado, A., Navas, N., Del Olmo, M. and Vilchez, J.L. (1998) Determination of Bisphenol A in Water by Micro Liquid-Liquid Extraction Followed by Silylation and Gas Chromatography-Mass Spectrometry Analysis. Journal of Chromatographic Science, 36, 565-569.
- Casajuana, N. and Lacorte, S. (2003) Presence and Release of Phthalic Esters and Other Endocrine Disrupting Compounds in Drinking Water. Chromatographia, 57, 649-655. http://dx.doi.org/10.1007/BF02491744
- Liu, R., Zhou, J.L. and Wilding, A. (2004) Simultaneous Determination of Endocrine Disrupting Phenolic Compounds and Steroids in Water by Solid-Phase Extraction-Gas Chromatography-Mass Spectrometry. Journal of Chromatography A, 1022, 179-189. http://dx.doi.org/10.1016/j.chroma.2003.09.035
- Tay, W.H., Yap, S.L. and Wong, C.S. (2014) Electrical Characteristics and Modeling of a Filamentary Dielectric Barrier Discharge in Atmospheric Air. Sains Malaysiana, 43, 583
- El-Zeer, D.M., Salem, A.A., Rashed, U.M., Abd Elbaset, T.A. and Ghalab, S. (2014) A Comparative Study between the Filamentary and Glow Modes of DBD Plasma in the Treatment of Wool Fibers. International Journal of Engineering Research and Applications, 4, 401-410.
- Konelschatz, U., Eliasson, B. and Egli, W. (1997) Dielectric-Barrier Discharges. Principle and Applications. Journal de Physique I11 d’octobre, 7, C4-47
- Kostov, K.G., Honda, R. Y., Alves, L.M.S. and Kayama, M.E. (2009) Characteristics of Dielectric Barrier Discharge Reactor for Material Treatment. Brazilian Journal of Physics, 39, 322. http://dx.doi.org/10.1590/S0103-97332009000300015
- Síra, M., Trunec, D., St’ahel, P., Bursíková, V. and Navrátil, Z. (2008) Surface Modification of Polycarbonate in Homogeneous Atmospheric Pressure Discharge. Journal of Physics D: Applied Physics, 41, Article ID: 015205. http://dx.doi.org/10.1088/0022-3727/41/1/015205
- Kokkoris, G., Vourdas, N. and Gogolides, E. (2008) Plasma Etching and Roughening of Thin Polymeric Films: A Fast, Accurate, in Situ Method of Surface Roughness Measurement. Plasma Processes and Polymers, 5, 825-833. http://dx.doi.org/10.1002/ppap.200800071
- Vargo, T.G., Gardella, J.A. and Salvati, L. (1989) Multitechnique Surface Spectroscopic Studies of Plasma Modified Polymers III. H2O and O2/H2O Plasma Modified Poly(Methyl Methacrylate)s. Journal of Polymer Science Part A: Polymer Chemistry, 27, 1267-1286. http://dx.doi.org/10.1002/pola.1989.080270413
- Yun, Y.I., Kim, K.S., Uhm, S.-J., Kkatua, B.B., Cho, K., Kim, J.K. and Park, C.E. (2004) Aging Behavior of Oxygen Plasma-Treated Polypropylene with Different Crystallinities. Journal of Adhesion Science and Technology, 18, 1279-1297. http://dx.doi.org/10.1163/1568561041588200
- Garamoon, A.A., Elakshar, F.F., Nossair, A.M. and Kotp, E.F. (2002) Experimental Study of Ozone Synthesis. Plasma Sources Science and Technology, 11, 254. http://dx.doi.org/10.1088/0963-0252/11/3/305
- Garamoon, A.A., Elakshar, F.F. and Elsawah, M. (2009) Optimizations of Ozone Generator at Low Resonance Frequency. The European Physical Journal Applied Physics, 48, 21002. http://dx.doi.org/10.1051/epjap/2009144
- Eliasson, B., Hirth, M. and Kogelschatz, U. (1987) Ozone Synthesis from Oxygen in Dielectric Barrier Discharges. Journal of Physics D: Applied Physics, 20, 1421. http://dx.doi.org/10.1088/0022-3727/20/11/010
NOTES

*Corresponding author.


