World Journal of Cardiovascular Surgery
Vol.05 No.10(2015), Article ID:60794,5 pages
10.4236/wjcs.2015.510017
Surgical Method for Facilitation of Aortic Valve Replacement: Intraoperative Application of External Pressure for Increasing the Transverse Axis of Aortic Annulus
Şahin Bozok1*, Mert Kestelli2, Aykut Şahin3, Hakan Kara4, Tevfik Güneş5, Berkan Özpak1, Gökhan Ilhan1, Serdar Bayrak2
1Department of Cardiovascular Surgery, Recep Tayyip Erdogan University Faculty of Medicine, Training and Research Hospital, Rize, Turkey
2Department of Cardiovascular Surgery, Izmir Katip Celebi University Faculty of Medicine, Atatürk Training and Research Hospital, Izmir, Turkey
3Department of Cardiovascular Surgery, Osmangazi University Faculty of Medicine, Eskişehir, Turkey
4Department of Cardiovascular Surgery, Ada Hospital, Giresun, Turkey
5Department of Cardiovascular Surgery, Pamukkale University Faculty of Medicine, Denizli, Turkey
Email: *sahinboz@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 September 2015; accepted 27 October 2015; published 30 October 2015
ABSTRACT
Background: Surgical method was introduced for enhancement of prosthetic valve insertion and overcoming difficulties ensourcing from prosthesis-patient mismatch. Methods: Twenty-two patients that underwent aortic valve replacement between January 2005 and July 2009 were included in this prospective study. In these patients, the insertion of prosthesis larger than the annulus diameter was attempted after the application of an external pressure that increased the transverse axis diameter of the aortic annulus. The postoperative results and complications were assessed. Results: This surgical method was performed on 22 patients (16 males, 6 females, mean age: 52.2 ± 15.8 years) during the valve replacement. In 12 patients (55%), replacement of proper sized aortic valve compliant to their surface area was accomplished, while the insertion of a proper valve could not be achieved in 10 (45%) of the patients. No perioperative mortality or complications related to the procedure were reported. Conclusion: Increasing the transverse diameter of aortic valve may not only facilitate the insertion of a prosthetic valve but also aid in overcoming prosthesis-patient mismatch. Further studies on larger series are necessary to document the actual effectively and precise selection criteria for application of this method.
Keywords:
Aortic Valve, Replacement, Prosthesis, Mismatch, Aortic Annulus

1. Introduction
Surgical treatment of aortic stenosis aims to correct the valvular pathology and relieve the pressure load on the left ventricle resulting in the reversal of pathologic anatomical changes and restoration of function [1] . Regression of left ventricular hypertrophy and other related anatomical changes after aortic valve replacement (AVR) seems to affect the long-term survival rates significantly [2] .
Valve prosthesis-patient mismatch (PPM) occurs when the effective orifice area of the inserted prosthetic valve is too small relative to body surface area. PPM is defined as a valve effective orifice area indexed for body surface area equal to or greater than 0.8 to 0.9 cm2/m2 [3] . This is a frequent problem in patients undergoing AVR (20% to 70% prevalence) that results in generation of high transvalvular gradients through normally functioning prosthetic valves [4] - [6] . Residual transprosthetic pressure gradients are important because an increased gradient will evidently result in an increased left ventricle work-load, jeopardizing the regression of left ventricle mass after AVR [3] . Mismatch between the orifice surface of the mechanical valve and the patient’s body surface area remains as a challenge in aortic valve surgery. Despite the definition of minimum valve sizes that could be inserted according to the surface area, unfortunately the replacement of a proper valve with respect to the surface area was not always feasible [3] [5] [6] .
In this manuscript, we suggest a novel surgical method that relies on the intraoperative manipulation of the aortic annulus by increasing the transverse diameter in order to eliminate the likelihood of patient-prosthesis mismatch. To our knowledge, this method has not been previously described in the literature (PubMed).
2. Patients and Methods
Study Design: This study was performed in the cardiovascular surgery department of a tertiary care center after the approval of Institutional Review Board. Twenty-two patients (16 males, 6 females) underwent AVR between January 2005 and July 2009 were prospectively evaluated. Routine blood tests and transthoracic echocardiography were performed preoperatively in all patients, while cardiac catheterization and coronary angiography were preserved for those at risk for coronary artery disease and multiple valve disease.
Surgical Method: All the patients were operated under general anesthesia using median sternotomy. Standard aortic arterial and uni-caval venous cannulation was performed on all patients, while venous cannulation was performed as bi-caval for candidates of mitral valve intervention. All the patients were cooled down to be 28˚C - 30˚C with a pump. Following the cross clamping, initial antegrade and subsequently retrograde isothermic hyperpotassemic blood cardioplegia was administered. The aorta was transversely opened after the cross clamping. Sling suturing was applied with 2/0 Tycron through each three commissure. Aortic valve was held from the leaflets and excised as to leave about 2 - 3 mm of the annulus. U sutures were passed with 2/0 Tycron sutures from the annulus with 2 mm intervals as the pledgets will be kept above the annulus. The sutures passed through the annulus were advanced through the mechanical valve suture ring. The horizontal axis of the mechanical valve was introduced into the annulus perpendicular to the horizontal axis of the aortic annulus. After fixation of first suture from where the aortic left coronary cusp adhered to the annulus, the valve was located and the suture in where the right coronary cusp adhered to the annulus was fixed. After completion of all the sutures, mechanical valve leaflets were checked, and the aortotomy was closed.
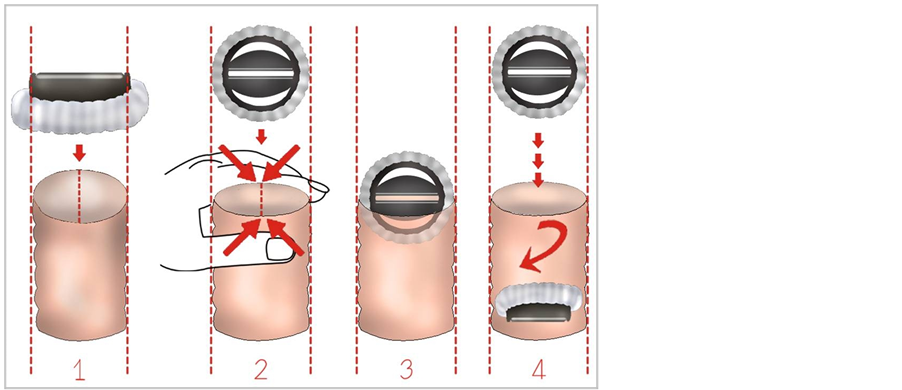
This novel surgical method, illustrated in Figure 1, is theoretically based on the insertion of the mechanical valve greater than the annulus diameter to the annulus owing to the application of an external pressure that increases the transverse axis.
All the patients received dopamine (Dopadren®, Vem Ilac, Istanbul, Turkey) 10 μg/kg/min and dobutamine (Dobutamin Konsantre®, Meditera Ilac, Izmir, Turkey) 10 μg/kg/min infusions before the cardiopulmonary bypass was finished. Intra-aortic balloon pump was used when required. The patients were fully monitorized under
Figure 1. Facilitated insertion of the aortic valve prosthesis by increasing the transverse diameter of the annulus by application of external pressure.
the mechanical ventilation during the intensive care and there was no perioperative mortality. ACE inhibitor, diuretics and anti-coagulant treatment (warfarin sodium 5 mg) were administered to all patients at the 1st postoperative day. Warfarin sodium (Coumadin®, Eczacibasi, Istanbul, Turkey) dose was set according to prothrombin time and international normalized ratio values measurements. Prothrombin time and international normalized ratio values were adjusted as to be 2 - 2.5 times of the normal values.
3. Results
The mean age of the study group was 52.2 ± 15.8 (range: 21 - 76) years. The co-morbidities reported included hypertension (n = 11), diabetes mellitus (n = 3), chronic pulmonary disease (n = 2), cerebrovascular disease (n = 2) and history of smoking (n = 10). Mitral valve replacement and concurrent coronary artery bypass were performed on five and two patients respectively.
The patients’ mean surface area was 1.75 ± 0.18 (range: 1.56 - 2.06) m2 and the average size of the valves inserted was no 23.45 ± 1.84 (range: 19 - 27) mm. Body surface areas of patients and data regarding aortic valve sizes are demonstrated in Table 1. In 12 patients (55%), replacement of proper-sized aortic valve compliant to their surface area was accomplished, while insertion of a proper valve could not be achieved in 10 (45%) of the patients.
Neither any complications related to the surgical procedure were encountered during the AVR, nor any perioperative mortality occurred. Four of the patients died due to heart failure during the long-term follow-up period after a minimum of two-year time interval.
4. Discussion
The term “prosthesis-patient mismatch” was described as the situation in which “the effective prosthetic valve area, after insertion into the patient, is less than that of a normal human valve” by Rahimtoola in 1978 [7] . In other words, PPM is expected to occur when the effective orifice area of the implanted prosthetic valve is too small in relation to the patient’s body size, despite the normal prosthesis function [2] [6] [8] .
Actually, many patients receiving a prosthetic aortic valve will have some degree of PPM, since the sewing ring, struts and leaflets of prostheses produce a relative obstruction to blood flow [2] [5] .
The phenomenon of PPM is mainly attributed to two main reasons. First, patients with aortic valve disease frequently exhibit annulus calcification and fibrosis in addition to the left ventricle hypertrophy, which reduce the size of the aortic annulus [9] - [11] . In these circumstances, prosthesis smaller than calculated with respect to the patient’s body surface area-should be implanted. Second, because the stented prosthesis has its own annulus, the effective orifice area after the implantation is actually smaller than that of a normal native valve. Factors that may predict PPM preoperatively are as follows: larger body surface area, high body mass index, older age,
Table 1. Body surface areas, required and inserted aortic valve sizes in our series.
smaller prosthesis size, and valvular stenosis as the predominant lesion before the operation [11] - [13] .
Patient-prosthesis mismatch occurs more frequently in patients with stenotic native valves and in older patients. This is consistent with the overall concept, because patients with stenotic native valves generally have a smaller valvular annulus than those with regurgitant valves, while calcific aortic stenosis is by far the most prevalent lesion in older patients undergoing AVR [5] - [7] .
General practice to PPM is the insertion of a closest smaller valve than the measured size of the annulus. A rapid and complete regression of the left ventricle hypertrophy can happen as a result of the superior hemodynamic performance and lower transvalvular gradient with the large prostheses. This subsequently provides a clinical recovery and prolongs the life expectancy. In contrary, small prostheses may be threatening in especially early and late periods of life and during exercise by creating an obstruction through the left ventricle outlet [11] - [16] . It was demonstrated that both prosthetic and bioprosthetic valves are narrower than the natural valves, and although function normally, they may lead to some degree of a gradient [17] [18] . These gradient values further increase in the cases with a small annulus by using valves with a small size [19] . To allow the usage of large valves, the annulus needs to be expanded before the replacement procedure [8] [15] . According to David’s definition of the minimum required valve sizes to be inserted with respect to the surface area, size no 21 is the minimum for a surface area < 1.5 m2, size no. 23 between 1.5 and 1.7 m 2 , size no. 25 between 1.7 and 1.9 m 2 and, size no. 27 for the surface area > 1.9 m 2 [3] .
Aortic annulus diameter decreases by 16% from systole to diastole [15] [20] . In our study, we considered the decrease of annulus diameter at the diastole and therefore, AVR was performed during the diastolic arrest, where the annulus was decreased by 16%. The annulus diameter measured during the valve replacement was smaller by 16% than the diameter pre-operation. Furthermore, intra-aortic pressure is zero when the valve is being replaced, hence it can be foreseen that the aortic annulus will be stretched a little more under the normal pressure after the replacement.
Limitations of our study are relatively small size of our series and lack of definite criteria for selection of patients for this method. However, we hope that this study will pioneer not only further studies on this method but also development of alternative options to eliminate PPM.
5. Conclusion
In conclusion, we believe one could act more courageously for the insertion of a larger valve than the aortic diameter measured during the operation. Changing the form of the aortic annulus, increasing the transverse diameter as well is an applicable and successful method that can aid in the insertion of the prosthesis.
Cite this paper
ŞahinBozok,MertKestelli,AykutŞahin,HakanKara,TevfikGüneş,BerkanÖzpak,GökhanIlhan,SerdarBayrak, (2015) Surgical Method for Facilitation of Aortic Valve Replacement: Intraoperative Application of External Pressure for Increasing the Transverse Axis of Aortic Annulus. World Journal of Cardiovascular Surgery,05,108-113. doi: 10.4236/wjcs.2015.510017
References
- 1. Monrad, E.S., Hess, O.M., Murakami, T., Nonogi, H., Corin, W.J. and Krayenbuehl, H.P. (1988) Time Course of Regression of Left Ventricular Hypertrophy after Aortic Valve Replacement. Circulation, 77, 1345-1355. http://dx.doi.org/10.1161/01.CIR.77.6.1345
- 2. Ali, A., Patel, A., Ali, Z., Abu-Omar, Y., Saeed, A., Athanasiou, T. and Pepper, J. (2011) Enhanced Left Ventricular Mass Regression after Aortic Valve Replacement in Patients with Aortic Stenosis Is Associated with Improved Long-Term Survival. The Journal of Thoracic and Cardiovascular, 142, 285-291. http://dx.doi.org/10.1016/j.jtcvs.2010.08.084
- 3. David, T.E. (1997) Complex Operations of the Aortic Root. In: Edmunds, L.H., Ed., Cardiac Surgery in the Adult, McGraw-Hill, New York, 939-958.
- 4. Okamura, H., Yamaguchi, A., Tanaka, M., Naito, K., Kimura, N., Kimura, C., et al. (2009) The 17-mm St. Jude Medical Regent Valve Is a Valid Option for Patients with a Small Aortic Annulus. The Annals of Thoracic Surgery, 87, 90-94. http://dx.doi.org/10.1016/j.athoracsur.2008.09.051
- 5. Jilaihawi, H., Chin, D., Spyt, T., Jeilan, M., Vasa-Nicotera, M., Bence, J., et al. (2010) Prosthesis-Patient Mismatch after Transcatheter Aortic Valve Implantation with the Medtronic-Corevalve Bioprosthesis. European Heart Journal, 31, 857-864. http://dx.doi.org/10.1093/eurheartj/ehp537
- 6. Reagan, B.W. and Kerut, E.K. (2005) Patient-Prosthetic Aortic Valve Mismatch: Role of the Echocardiographer. Echocardiography, 22, 365-366.
http://dx.doi.org/10.1111/j.1540-8175.2005.40015.x - 7. Rahimtoola, S.H. (1978) The Problem of Valve Prosthesis-Patient Mismatch. Circulation, 58, 20-24. http://dx.doi.org/10.1161/01.CIR.58.1.20
- 8. Tuzcu, E.M., Özkan, A. and Kapadia, S.R. (2011) Prosthesis-Patient Mismatch in the Transcatheter Aortic Valve Replacement Era. Journal of the American College of Cardiology, 58, 1919-1922.
http://dx.doi.org/10.1016/j.jacc.2011.08.037 - 9. Ozsoyler, I., Lafci, B., Emrecan, B., Kestelli, M., Bozok, S., Ozbek, C., et al. (2006) Aortic Valve Replacement in True Severe Aortic Stenosis with Low Gradient and Low Ejection Fraction. The Heart Surgery Forum, 9, E681-E685. http://dx.doi.org/10.1532/HSF98.20061039
- 10. Helvac1, A. and Helvac1, F. (2014) Clinical Approach to Severe Aortic Stenosis with Low Flow and Low Gradient. Turk Gogus Kalp Dama, 22, 694-701.
http://dx.doi.org/10.5606/tgkdc.dergisi.2014.8892 - 11. Gonzalez-Juanatey, J.R., Fernandez, M.V., Sampedro, F.G., Garcia-Acuna, J.M., Garcia-Bengoechea, J.B., Cendon, A.A., et al. (1999) Hemodynamic Performance of Aortic Pericardial Bioprostheses and Bileaflet Prostheses at Rest and during Exercise: Implications for the Surgical Management of Patients with Small Aortic Roots. Heart, 82, 149-155. http://dx.doi.org/10.1136/hrt.82.2.149
- 12. Gillinov, A.M., Blackstone, E.H. and Rodriguez, L.L. (2003) Prosthesis-Patient Size: Measurement and Clinical, Implications. The Journal of Thoracic and Cardiovascular Surgery, 126, 313-316.
http://dx.doi.org/10.1016/S0022-5223(02)73223-6 - 13. Dumesnil, J.G. and Pibarot, P. (2004) Prosthesis Size and Prosthesis-Patient Size Are Unrelated to Prosthesis-Patient Mismatch. The Journal of Thoracic and Cardiovascular Surgery, 127, 1852-1854. http://dx.doi.org/10.1016/j.jtcvs.2003.11.073
- 14. Akyuz, M., Celik, E. and Kestelli, M. (2012) Solutions for Transcatheter Aortic Valve Implantation. Turk Gogus Kalp Dama, 20, 965. http://dx.doi.org/10.5606/tgkdc.dergisi.2012.195
- 15. Cakici, M., Durdu, S., Inan, B., Yazicioglu, L., Sirlak, M., Eryilmaz, S., et al. (2013) Changes in Left Ventricular Function and Geometry after Aortic Valve Replacement in Patients with Severe Aortic Stenosis. Turk Gogus Kalp Dama, 21, 284-293. http://dx.doi.org/10.5606/tgkdc.dergisi.2013.6999
- 16. Tasdemir, O. (2005) Small Aortic Annulus and Mechanic Aortic Valves. Anadolu Kardiyoloji Dergisi, 5, 34-35.
- 17. van den Brink, R.B., Verheul, H.A., Visser, C.A., Koelemay, M.J. and Dunning, A.J. (1992) Value of Exercise Doppler Echocardiography in Patients with Prosthetic or Bioprosthetic Cardiac Valves. The American Journal of Cardiology, 69, 367-372. http://dx.doi.org/10.1016/0002-9149(92)90235-Q
- 18. Wiseth, R., Levang, O.W., Tangen, G., Rein, K.A., Skjaerpe, T. and Hatle, L. (1993) Exercise Hemodynamics in Small (£ 21 mm ) Aortic Valve Prostheses Assessed by Doppler Echocardiography. American Heart Journal, 125, 138-146. http://dx.doi.org/10.1016/0002-8703(93)90066-I
- 19. Demesnil, J.G. and Yoganathan, A.P. (1992) Valve Prosthesis Hemodynamics and the Problem of High Transprosthetic Pressure Gradients. European Journal of Cardio-Thoracic Surgery, 6, S34-S38. http://dx.doi.org/10.1016/1010-7940(92)90019-t
- 20. Thubrikar, M.J., Nolan, S.P., Aouad, J. and Deck, J.D. (1986) Stress Sharing between the Sinus and Leaflets of Canine Aortic Valve. The Annals of Thoracic Surgery, 42, 434-440.
http://dx.doi.org/10.1016/S0003-4975(10)60554-1
NOTES

*Corresponding author.