Open Journal of Animal Sciences
Vol.2 No.3(2012), Article ID:21363,8 pages DOI:10.4236/ojas.2012.23020
In vitro ruminal fermentation of leaves from three tree forages in response to incremental levels of polyethylene glycol
![]()
1Department of Food Production, Faculty of Science and Agriculture, University of The West Indies, St. Augustine, Trinidad and Tobago; *Corresponding Author: andell_e@hotmail.com
2The Open Tropical Forage-Animal Production Laboratory (OTP-APL), Department of Food Production, Faculty of Science and Agriculture, University of the West Indies, St. Augustine, Trinidad and Tobago
3School of Veterinary Medicine, Faculty of Medical Sciences, Mt Hope, Trinidad and Tobago
Received 2 April 2012; revised 6 May 2012; accepted 17 May 2012
Keywords: Tannin-Binding Agents; In Vitro Ruminal Gas Production; Organic Matter Digestibility; Tannin Bioactivity; Optimum Inclusion Rate
ABSTRACT
Polyethylene glycol (PEG), a phenol binding agent has been used extensively to measure the biological activity of tannins in forage species. The optimum inclusion rate of PEG, per unit weight of sample varies from species to species. Determining optimum inclusion levels can prevent wastage and reduce the cost of diagnosing the biological activity of tannins, especially in developing countries. This study was designed to determine the optimum PEG inclusion levels required to completely ameliorate in vitro ruminal bioactivity of tannins in leaves from Leucaena leucocephala, Gliricidia sepium and Trichanthera gigantea using the Reading Pressure Technique. Fermentation parameters were generated by fitting gas production data to the Orskov and McDonald (1979) non-linear equation: . An asymptotic response to incremental levels of PEG was observed with cumulative gas production at 48 h post inoculation. The minimum level of PEG required to maximize in vitro ruminal fermentation of tree leaves was found to be 200 mg PEG/g DM for all tree species. Gas production rate constant for the insoluble fraction (c) showed an increase (P < 0.05) upon PEG addition for all species. In vitro organic matter degradability (iOMD) declined (P < 0.05) in the leaves of G. sepium (565 - 540 g/kg DM) whereas, there was an increase (P < 0.05) in the iOMD of T. gigantea leaves (328 - 340 g/kg DM) upon PEG addition. Partitioning factor (PF) declined (P < 0.05) upon PEG addition for all species. Predicted metabolizable energy was highest (P < 0.05) in the leaves of G. sepium (8.7 MJ/kg DM) and lowest in T. gigantea leaves (5.4 MJ/kg DM) upon PEG addition. It is concluded that a PEG inclusion level of 200 mg/g DM sample is sufficient for the diagnosis of in vitro ruminal tannin biological activity in leaves of the three tree species.
. An asymptotic response to incremental levels of PEG was observed with cumulative gas production at 48 h post inoculation. The minimum level of PEG required to maximize in vitro ruminal fermentation of tree leaves was found to be 200 mg PEG/g DM for all tree species. Gas production rate constant for the insoluble fraction (c) showed an increase (P < 0.05) upon PEG addition for all species. In vitro organic matter degradability (iOMD) declined (P < 0.05) in the leaves of G. sepium (565 - 540 g/kg DM) whereas, there was an increase (P < 0.05) in the iOMD of T. gigantea leaves (328 - 340 g/kg DM) upon PEG addition. Partitioning factor (PF) declined (P < 0.05) upon PEG addition for all species. Predicted metabolizable energy was highest (P < 0.05) in the leaves of G. sepium (8.7 MJ/kg DM) and lowest in T. gigantea leaves (5.4 MJ/kg DM) upon PEG addition. It is concluded that a PEG inclusion level of 200 mg/g DM sample is sufficient for the diagnosis of in vitro ruminal tannin biological activity in leaves of the three tree species.
1. INTRODUCTION
Phenol binding agents, such as polyvinyl polypyrrolidone (PVPP), polyethylene glycol (PEG) and polyvinyl pyrrolidone (PVP) have been used extensively to investigate the in vitro ruminal biological activity of tannins in browse species [1,2]. These compounds usually form stable complexes with tannins hence preventing the binding between tannins and proteins. Incubating plant samples in the presence or absence of a phenol binding agent constitutes the in vitro tannin bioassay. The difference in fermentation characteristics between treated and untreated substrates gives an idea of the biological effects of tannins in rumen fermentation [2]. Polyethylene glycol is the more widely used binding agent owing to its greater affinity to bind tannins [3]. As a synthetic polymer, PEG inactivates tannins by forming tannin-PEG complexes [3]. In addition, PEG treatment can reverse already formed tannin-substrates complexes, thus making substrates available for fermentation [4].
Although several authors have reported positive effects of PEG inclusion on in vitro ruminal fermentation of tanniniferous forages [5,6], its use in vivo by small holder farmers may be limited by cost and availability. It is therefore prudent to ensure that only the optimum inclusion rates are used to prevent oversupply in diets. Recommendations on inclusion rates of PEG for complete tannin neutralization vary widely. According to [3], PEG supplementation levels of 1 g PEG per gram of sample for in vitro studies while, [5] indicated that 15 mg PEG would be sufficient to neutralize 75 g CT/g sample in the diets of animals. It was suggested by [7] that 2 g of PEG to 5 g dried and ground foliage can completely annul the suppressive effects of tannins in vitro. These inclusion rates may not be universally applicable since the efficiency of PEG binding to tannins can vary across forage species owing to the different chemical structure and molecular weight of tannins [3]. As such, an investigation of the optimum inclusion rate of PEG should be conducted for forages that vary sufficiently from those used in previous studies, especially if their growth environment differs significantly.
Leucaena leucocephala, G. sepium and T. gigantea can be used to supplement the poor quality diets of ruminant animals in Trinidad and Tobago. Crude protein (CP) content of leaves from these species range from 185 to 318 g/kg DM, making them potential protein supplements [8]. It was reported that the supplementary effect of legumes on the in vivo digestibility of grasses is due to the legume overcoming a Nitrogen deficiency [9]. Their use, however, may be limited by anti nutritional compounds such as tannins. It was indicated by [10] that condensed tannins interrupt microbial attachment to feed particles and inhibit ruminal fermentation. Fermentation can be further affected as tannins exert deleterious effects on microbial and enzymatic activities [1]. According to [11] the toxic effects of tannins can be due to tannins inhibiting enzyme activity and causing substrates and metal ions to be unavailable to microbes. If microbial activity is hindered, the extent of degradation in the rumen would decline dramatically. As a result, the utilization of crude protein (CP) in forage species by ruminants could be limited by elevated levels of biologically active condensed tannins [5].
There is a lack of information about the optimum inclusion level of PEG for tannin amelioration in L. leucocephala, G. sepium and T. gigantea foliage being grown for ruminants in Trinidad and Tobago. This paper therefore seeks to determine the optimum inclusion level of PEG for in vitro-based diagnosis of tannin bioactivity in G. sepium, L. leucocephala and T. gigantea leaves using the Reading Pressure Technique [12].
2. MATERIALS AND METHODS
2.1. Study Site
Leaf samples were obtained from established tree species at the University of the West Indies Field Station (UFS). The UFS (Lat 10˚38'N Long 61˚23'W) has a relatively flat topography with an altitude of 15.2 meters above mean sea level. Average annual rainfall is 1782.9 mm with an average monthly temperature of 27˚C. The soil type is river estate loam. The soil is free draining with a pH range of 5.0 - 6.2.
2.2. Sample Preparations
Fresh leaf materials (leaves with petioles) were harvested from forage tree species (L. leucocephala, G. sepium, and T. gigantea) that were trimmed to a height of 1 meter at UFS. Harvesting was done in the morning manually by cutting branches at a distance of 1 m from the growing tip for (G. sepium) and 0.5 m for (T. gigantea and L. leucocephala), 8 weeks after the trees had been trimmed to a 1 meter height. Leaves from six individual trees of each species were harvested, weighed and stored in brown paper bags separately. Leaf samples were immediately transported to the laboratory and oven dried to a constant weight at 65˚C. The dried samples were then milled to pass through a 1 mm sieve using a Wiley Mill (GLEN CRESTON LTD, MIDDLESEX, UK) and kept in separate brown paper bags pending chemical analysis and in vitro ruminal fermentation.
2.3. Chemical Analyses
Chemical analyses were carried out as part of an earlier study [8]. Dry matter, organic matter, crude protein, neutral detergent fibre, acid detergent fibre, acid detergent lignin, soluble and insoluble condensed tannin content of leaves were determined. The chemical composition of the leaves is presented in Table 1 below to further describe the substrates used in this study.
2.4. Reading Pressure Technique Procedure
The response of in vitro ruminal microbial fermentation to incremental levels of PEG was assessed using the Reading Pressure Technique (RPT) [12]. Milled leaf substrates (1 g) were weighed into 125 ml serum bottles. Using a measuring cylinder, 90 ml reduced buffer (RPT mix) was added to each serum bottle. Serum bottles without samples (blanks) were also included to allow correction for gas produced from rumen liquor. After addition of the buffer, the flasks were sealed and stored at room temperature (20˚C) before being transferred into the incubators, set at 39˚C, 8 hours (h) before inoculation with rumen fluid. Ruminal fluid was collected at 8:00 am. The donor was a crossbred Holstein heifer that was offered tanner grass, G. sepium, L. leucocephala, T. gigantea leaves and dairy concentrate (MASTER MIX FEEDS LTD, TRINIDAD). Rumen digesta from multiple sites within the rumen was collected by hand and the rumen fluid squeezed into a prewarmed insulated flask. It was then transported to the laboratory and strained through two layers of warm cheese cloth. The strained rumen fluid was held under carbon dioxide at 39˚C. A total of 126 serum bottles were inoculated within an hour of obtaining rumen fluid and incubated at 39˚C.
2.5. Polyethylene Glycol Inclusion
Polyethylene glycol treatment levels of 0, 50, 100, 150, 200, 250, 300, 500, 1000 mg/g DM leaf substrate, were prepared by dissolving PEG (Molecular weight 6000, FISHER SCIENTIFIC COMPANY, UK), in eight sets of buffer while, no PEG was added to the ninth set to create a zero PEG level. Buffers (90 ml) were then dispensed into serum bottles according to assigned PEG treatments.
2.6. Gas Measurements
Headspace gas pressure was measured at 2, 4, 6, 8, 10, 12, 15, 19, 24, 30, 36, 48, 72 and 96 h post-inoculation using a pressure transducer. Gas pressure readings pressure per square inch (psi) was converted to gas volume (ml) using the following relationship between gas pressure and gas volume that was predetermined for the site:

where Gp = is the predicted gas volume (ml) and psi is the pressure transducer reading at time t.
2.7. Estimation of Degraded Substrate, Partitioning Factors and Metabolizable Energy
In vitro organic matter degradability (iOMD) at 48 h was determined by recovering the fermentation residues by filtration through sintered-glass crucibles (100 - 160 µm porosity, PYREX, STONE, UK) under vacuum. Fermentation residues were dried at 105˚C overnight and incinerated in a muffle furnace at 550˚C for 12 h. Loss in weight after incineration was used as a measure of undegradable OM. The iOMD was calculated as the difference between OM content of the substrate and its undegradable OM. Partitioning factors (PF), a measure of fermentation efficiency, were calculated as a ratio of iOMD (mg) and cumulative gas production (ml/g OM) at 96 h post-inoculation. Metabolizable energy (ME) (MJ/kg DM) content of leaf substrates was predicted from 96 h organic matter degradability using the following equation [13] for organic matter, as follows:

where iOMD = g digestible organic matter per kg dry matter (DM).
3. STATISTICAL ANALYSIS
The optimum PEG inclusion rate was determined from predicted responses by identifying the point where fermentation response begins to increase at a decreasing rate. A non-linear regression model was fitted to 48-h cumulative gas release data using the Curve Fitter [14] to describe the process underlying the response to incremental levels of PEG. The non-linear regression model used was of the form:

where: Y = 48 h cumulative gas produced at PEG inclusion level x; a = cumulative gas release of untreated leaf substrate (x = 0); b = cumulative gas release in response to incremental levels of PEG; c = rate at which cumulative gas production respond to increase in rate of PEG treatment; x = level of PEG treatment.
48 h cumulative gas production data at zero and the optimum PEG inclusion rates were fitted to the model of [15]:

where: y = gas produced at time t; a = gas production from the immediately soluble fraction (ml); b = gas production from the insoluble fraction (ml); c = gas production rate constant for the insoluble fraction (ml/h); t = incubation time (h); tl = lag time.

Table 1. The effect of species and cutting interval (weeks) on the chemical composition (g/kg DM) of G. sepium, L. leucocephala and T. gigantea leaves [8].
General linear models (GLM) procedures of Minitab (Minitab 2000) were used for the analysis of variance of data for 48 h cumulative gas production, rate of gas production, iOMD, partitioning factors, predicted metabolizable energy (ME) and fitted parameters (a, b and c) at 0 and at the optimum inclusion level of PEG. A 3 × 2 factorial treatment (3 species and 2 PEG levels) arrangement in a completely randomised design (CRD) was employed using the following model:
Fermentation parameters = µ + Species + PEG
+ Species × PEG
+ Error.
4. RESULTS
Response of Fermentation Parameters to PEG Treatment of Leaves
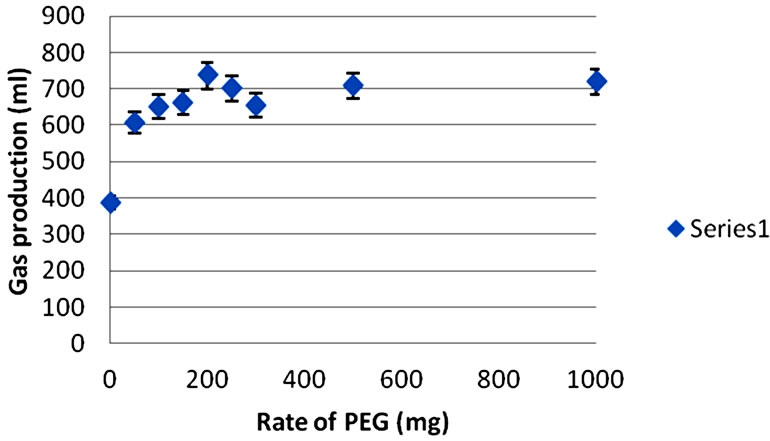
Figure 1 shows the 48 h cumulative gas response to the rate of PEG inclusion for G. sepium, L. leucocephala, and T. gigantea leaves. Increasing the level of PEG caused an exponential rise to a maximum (asymptotic) response for all of the tree species.
The optimum level of PEG inclusion to maximize in vitro fermentation was 200 mg for all of the tree species. Trichanthera gigantea gave the highest percentage increase in 48 h gas production upon PEG addition (41%) whereas G. sepium gave the lowest percentage increase in gas production upon PEG addition (7%).
Table 2 shows the effect of optimum PEG treatment (200 mg) on the gas produced from the immediately soluble fraction (a), the insoluble fraction (b), gas production rate constant for the insoluble fraction (c), potential gas production (a + b) and organic matter digestibility (OMD) 48 hours post-incubation. Cumulative gas production upon PEG addition were: 736 ml/g OM (G. sepium), 557 ml/g OM (L. leucocephala), and 368 ml/g OM (T. gigantea). Gas production without PEG inclusion was highest in the leaves of G. sepium (687 ml/g OM) and lowest in T. gigantea (262 ml/g OM) leaves. Gas production from the immediately soluble fraction (a) showed a decrease (P < 0.05) from 28.2 to 17.6 ml/g OM upon PEG addition for T. gigantea. There was no difference (P < 0.05) in gas production from the immediately soluble fraction (a) upon PEG addition for G. sepium and L. leucocephala. Gas production from the immediately soluble fraction (a) was highest (P < 0.05) for T. gigantea (17.6 ml/g OM) and lowest for G. sepium (8.0 ml/g OM) upon PEG addition. There was no difference (P < 0.05) in gas production from the insoluble fraction (b) upon PEG addition for all species. Gas production from the insoluble fraction (b) upon PEG addition was highest (P < 0.05) for G. sepium (822 ml/g OM) and lowest for T.
 (a)
(a) (b)
(b)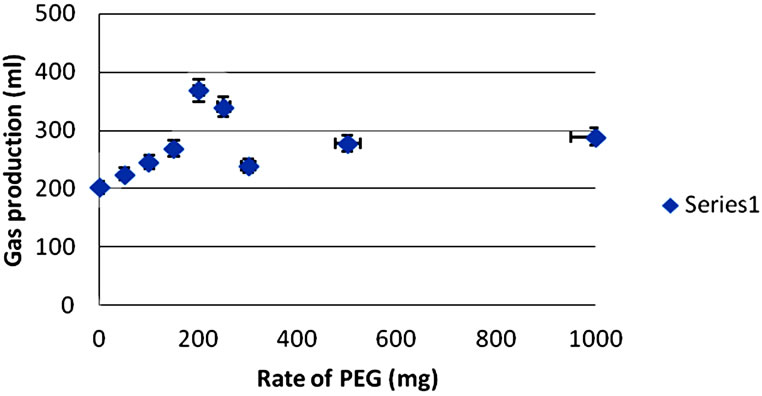 (c)
(c)
Figure 1. Cumulative gas production (48 h) in response to incremental levels of PEG from (a) G. sepium, (b) L. leucocephala and (c) T. gigantea leaves.

Table 2. The effect of optimum inclusion rate (mg) of polyethylene glycol on 48 h gas production kinetics partitioning factor (PF) (mg/l), predicted metabolizable energy (ME) (MJ/kg DM) and organic matter degradability (iOMD) of G. sepium, L. leucocephala and T. gigantea leaves.
gigantea (564 ml/g OM). Gas production rate constant for the insoluble fraction (c) showed an increase (P < 0.05) upon PEG addition for all species.
The percentage increase in gas production rate constant for the insoluble fraction (c) upon PEG addition was highest (P < 0.05) for T. gigantea (100%) and lowest for G. sepium (25%). Potential gas production (a + b) showed an increase (P < 0.05) when PEG was added to T. gigantea leaves (505 - 643 ml/g OM). However, there was no difference (P < 0.05) in potential gas production (a + b) in the leaves of G. sepium and L. leucocephala when PEG was added. Potential gas production (a + b) was highest (P < 0.05) for G. sepium (830 ml/g OM) and lowest for L. leucocephala (637 ml/g OM) upon PEG addition. There was an increase (P < 0.05) in the lag phase upon PEG addition for T. gigantea (–0.4 - 1.9 ml/g OM). However, there was no difference (P < 0.05) in the lag phase upon PEG addition for G. sepium and L. leucocephala. Trichanthera gigantea recorded the highest (P < 0.05) lag phase value (1.9 ml/g OM) upon PEG addition. The partitioning factor (PF) showed a decline (P < 0.05) upon PEG addition for all species. There was a decrease (P < 0.05) in the predicted metabolizable energy (ME) for G. sepium (from 9 to 8.7 MJ/kg DM), whereas, there was no difference (P < 0.05) in predicted ME in the leaves of L. leucocephala and T. gigantea upon PEG addition. Predicted metabolizable energy was highest (P < 0.05) in the leaves of G. sepium (8.7 MJ/kg DM) and lowest in T. gigantea leaves (5.4 MJ/kg DM) upon PEG addition. For PEG-treated substrates, iOMD was highest (P < 0.05) in the leaves of G. sepium (540 g/kg DM) and lowest in the leaves of T. gigantea (340 g/kg DM). There was a decline (P < 0.05) in iOMD of G. sepium (565 540 g/kg DM) upon PEG addition. In contrast, there was an increase (P < 0.05) in the iOMD of T. gigantea leaves (328 - 340 g/kg DM) upon PEG addition.
5. DISCUSSION
This study was designed to test the hypothesis that tannin inactivation in tree leaves responds to incremental PEG levels in an asymptotic fashion. Data generated support this hypothesis for all species (Figure 1). These cumulative gas production response profiles illustrate the asymptotic nature of the response of in vitro gas production to incremental levels of PEG treatments. Cumulative gas production after 48 hours of incubation increased with the level of PEG treatment with the response tending to curvilinear as the maximum effect is approached. The increase in cumulative gas production in response to PEG inclusion is supported by [3,5,10,16-18]. Further increases beyond 200 mg PEG/g leaf substrate did not result in significant increases in cumulative gas production. This corroborates findings reported by [19] in phenolic-rich tree fruits. The optimum level of PEG required to maximize in vitro gas production was estimated using the curve fitter programme [14] that illustrated the asymptotic response (Figure 1). For asymptotic curves, the point where the curve ceases to climb is difficult to identify, as a consequence, it is difficult to accurately determine the optimum PEG level for all species. Work done by [19], suggest that the minimum PEG level can be estimated from the fitted equations using the predicted responses and identifying where fermentation response begins to increase at a decreasing rate. Following this approach, optimum PEG inclusion levels were found to be 200 mg/g OM for all species. This was not expected as tannin activity tends to differ with species. Our previous work [8] demonstrated that colorimetrically assayed extractable condensed tannin levels in all species, except L. leucocephala, were negligible, however, chemical composition alone may not be a good indicator of tannin bioactivity [20]. There is also the possibility that PEG can bind to solvent-insoluble tannins [21] but these were not quantified in our earlier study.
Gas production without PEG inclusion was highest in the leaves of G. sepium (687 ml/g OM) and lowest in T. gigantea (262 ml/g OM) leaves (Table 2). The lower extent of in vitro fermentation of T. gigantea leaves could be attributed to their elevated fibre components (NDF, ADF) and lignin (ADL) contents compared to G. sepium leaves (Table 1). Fibre and lignin components have the ability to limit in vitro ruminal fermentation. It was reported by [22,23] that negative correlations exists between NDF, ADF, ADL and rate and extent of gas production (GP) in tannin containing browse species. The reduction in microbial activity could be in response to the lower proportions of cell contents and hence a lower concentration of readily available nutrients.
When fermentation parameters of substrates incubated with optimum PEG inclusion rate were compared to those of substrates incubated without PEG, T. gigantea recorded the highest percentage increase in gas production (41%) whereas G. sepium gave the lowest percentage increase in gas production (7%) upon PEG addition. This indicates that tannins in the leaves of T. gigantea are more biologically active in comparison to G. sepium. Though no tannins were detected in the leaves of G. sepium and T. gigantea, their response to PEG can be due to the fact that PEG can bind to tannins that may not be extracted by solvents [21]. The gas production rate constant for the insoluble fraction (c) showed a significant increase upon PEG addition for all species. This increase could be attributed to PEG liberating substrates from tannin complexes hence releasing a greater volume of nutrients for ruminal microorganisms [10,24]. It was noted by [10] that condensed tannins disrupt microbial attachment to feed particles and as a consequence, inhibit ruminal fermentation. This effect was most pronounced in T. gigantea leaves as there was a 100% increase in the rate of degradation upon PEG addition (Table 2). This observation was in contrast to [17] who reported that PEG 8000 did not influence the in vitro gas production rate constant for the insoluble fraction (c) of Quercus cerris leaves. This may be due to plant species difference and the difference in the molecular weight of PEG used as this can influence the affinity of binding to tannins. There was a significant increase in the lag phase (L) of T. gigantea leaves (–0.4 - 1.9 ml/g OM) upon PEG addition. A substantive explanation cannot be given thus far for this phenomenon. Potential gas production (a + b) showed an increase of 27% when PEG was added to T. gigantea leaves. This is supported by [5] in a study utilizing (PEG 8000) to evaluate its effect on in vitro gas production kinetics of tannin containing tree leaves. There was no difference (P < 0.05) in potential gas production (a + b) in the leaves of G sepium and L. leucocephala when PEG was added (Table 2). This may be due to the differences in CP concentrations (Table 1) as response to PEG treatment is dependent on the level of protein in the substrates. Also, PEG binding to tannins can vary across forage species owing to the different chemical structure and molecular weight of tannins [3,5]. There was no difference in gas production from the insoluble fraction (b) upon PEG addition for all of the tree species in this study (Table 2). This is probably due to the inability of PEG to liberate the nutrients that are bound to the structural components of leaf substrates. A significant decline in the partitioning factor was observed for all species upon PEG addition. This indicates that PEG inclusion reduced fermentation efficiency; a result of high gas production and lower OMD values. [10] indicated that reduced partitioning values can result from a lower partitioning of nutrients to microbial protein synthesis. As tannins bind to substrates nutrients are released at a much slower rate to the microbes [25]. There was a (0.4%) decrease in predicted metabolizable energy (ME) for G. sepium upon PEG addition. This might partly be explained by the decrease in fermentation efficiency upon PEG addition. There was 4% increase in the iOMD of T. gigantea leaves upon PEG addition (Table 2). This is in agreement with [5,6,18] who reported significant increases in OMD with PEG inclusion on tanniniferous tree species. This increase in degradability may be due to increases in both the rate and extent of digestion by microbes when PEG was added to the leaf substrates [26]. In vitro organic matter degradability gives an idea of the bioavailability of the soluble cell contents of forages. It can also be used to predict the ME concentration of the feed being analyzed. This is critical as provides the nutritionist with valuable information that can help in making inform decisions. In contrast, there was a significant decline in iOMD of G. sepium (from 565 to 540 g/kg DM) upon PEG addition. PEG-tannin complexes in the residues may have lead to an underestimation of fermentation values [4,3,27]. It is also possible that the anti nutritional activity of non-tannin phenolics that do not bind to PEG may reduce fermentation values as they may have a deleterious effect on microbes [19,27]. It is therefore possible, that G. sepium leaves may contain these nontannin phenolics. Nitrogen degradability is less affected by PEG-tannin complexes and can be used as an appropriate tool to measure degradability responses to PEG inclusion in tanniniferous forages. Additionally, the effect of PEG is limited by the level of protein in the leaf substrates. According to [28] the higher the protein level in the substrate, the lower is the PEG effect since protein does not be limit fermentation. The effect of tannins on fermentation is also reduced since microbes have access to the extra proteins. Indeed, the CP value of G. sepium was higher than that of T. gigantea (Table 1).
6. CONCLUSION
The optimum level of PEG required to maximize in vitro ruminal fermentation of leaves from G. sepium, L. leucocephala and T. gigantea was found to be 200 mg/g DM of sample. This optimum level may be limited to these three species as tannin biological activity varies from species to species. The increase in in vitro gas production kinetics and OMD of some species with PEG addition emphasizes the negative effects of tannins on degradability. Determining optimum PEG inclusion levels is critical as it makes it more cost effective to evaluate tanniniferous forages in developing countries.
7. ACKNOWLEDGEMENTS
We express sincere gratitude to the lab technicians of the Food Production Lab, Department of Food Production, University of the West Indies for lending their support and expertise. We would also like to thank Graduate Studies, University of the West Indies (St Augustine Campus) for providing the funding to purchase chemicals.
REFERENCES
- Silanikove, N., Nitsan, Z. and Perevolotsky, A. (1994) Effect of daily supplementation of polyethylene glycol on intake and digestion of tannin-containing leaves (Ceratonia siliqua) by sheep. Journal of Agricultural Food Chemistry, 42, 2844-2847. doi:10.1021/jf00048a035
- Mlambo, V., Mould, F.L., Smith, T., Owen, E., Sikosana, J.L.N. and Mueller-Harvey, I. (2009) In vitro biological activity of tannins from Acacia and other tree fruits: Correlations with colorimetric and gravimetric phenolic assays. South African Journal of Animal Science, 39, 131- 143. doi:10.4314/sajas.v39i2.44387
- Makkar, H.P.S., Blummel, M. and Becker, K. (1995) Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. British Journal of Nutrition, 73, 897-913. doi:10.1079/BJN19950095
- Mangan, J.L. (1988) Nutritional effects of tannins in animal feeds. Nutrition Research Reviews, 1, 209-231. doi:10.1079/NRR19880015
- Kamalak, A., Canbolat, O., Sahin, M., Gurbuz, Y., Ozkose, E. and Ozkan, C.O. (2005) The effect of polyethylene glycol (PEG 8000) supplementation on in vitro gas production kinetics of leaves from tannin containing trees. South African Journal of Animal Science, 35, 229-237.
- Karabulut, A., Canbolat, O. and Kamalak, A. (2006) The effect of PEG on in vitro organic matter digestibility and metabolizable energy of Lotus corniculatus L. Lotus Newsletter, 36, 7-10.
- Aharoni, Y., Gilboa, N. and Silanikove, N. (1998) Models of suppressive effect of tannins. Analysis of the suppressive effect of tannins on ruminal degradation by compartmental models. Animal Feed Science and Technology, 71, 251-267. doi:10.1016/S0377-8401(97)00147-8
- Edwards, A., Mlambo, V., Lallo, C.H.O. and Garcia, G.W. (2012) Yield, chemical composition and in vitro ruminal fermentation of the leaves of Leucaena leucocephala, Gliricidia sepium and Trichanthera gigantea as influenced by harvesting frequency. Journal of Animal Science Advances, 2, 321-331.
- McLeod, M.N. and Minson, D. (1969) The use of the in vitro technique in the determination of the digestibility of grass/legumes mixtures. Journal of the British Grassland Society, 24, 296-298. doi:10.1111/j.1365-2494.1969.tb01084.x
- Sallam, H.A.M.S., Bueno, I.C., Barbosa de Godoy, P., Nozella, E.F., Vitti, D.M.M.S. and Abdalla, A.L. (2010) Ruminal fermentation and tannin bioactivity of some browses using a semi-automated gas production technique. Tropical and Subtropical Agroecosystems, 12, 1- 10.
- Scalbert, A. (1991) Antimicrobial properties of tannins. Phytochemistry, 30, 3875-3883. doi:10.1016/0031-9422(91)83426-L
- Mauricio, R.M., Mould, F.L., Dhanoa, M.S., Owen, E., Channa, K.S. and Theodorou, M.K. (1999) A semiautomated in vitro gas production technique for ruminant feedstuff evaluation. Animal Feed Science and Technology, 82, 227-241.
- Mc Donald, P., Edwards, R.A., Greenhalgh, J.F.D. and Morgan, C.A. (2002) Animal nutrition. 6th Edition, Longman House, England.
- Datafit (2009) Data curve fitting (nonlinear regression) and data plotting software. Version 9.0, Oakdale Engineering, USA.
- Orskov, E.R. and McDonald, I. (1979) The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. Journal of Agricultural Science Cambridge, 92, 499-503. doi:10.1017/S0021859600063048
- Mlambo, V. (2002) Modifying the nutritional effects of tannins present in Acacia and other tree fruits offered as protein supplements to goats in Zimbabwe, Ph.D. Dissertation, Department of Agriculture, University of Reading, London.
- Canbolat, O., Kamalak, A., Ozkose, E., Ozkan, C.O., Sahin, M. and Karabay, P. (2005) Effect of polyethylene glycol on in vitro gas production, metobolizable energy and organic matter digestibility of Quercus cerris leaves. Livestock Research for Rural Development, 17, Accessed 10 February 2012. http://www.lrrd.org/lrrd17/4/canb17042.htm
- Mtui, D. J., Lekule, F.P., Shem, M.N., Ichinohe, T. and Fujihara, T. (2009) Comparative potential nutritive value of grasses, creeping legumes and multipurpose trees commonly in sub humid region in the eastern parts of Tanzania. Livestock Research for Rural Development, 21, accessed 19 December 2011. http://www.lrrd.org/lrrd21/10/mtui21158.htm
- Mlambo, V., Mould, F.L., Smith, T., Owen, E., Sikosana, J.L.N. and Mueller-Harvey, I. (2006) In vitro gas production and nitrogen degradability of tannin-containing tree fruits in response to incremental levels of polyethylene glycol. UNISWA Journal of Agriculture, 14, 120-131.
- Vitti, D.M.S.S., Abdalla, A.L., Bueno, I.C.S., Silva Filho, J.C., Costa, C., Bueno, M.S., Nozella, E.F., Longo, C., Vieira, E.G., Cabral Filho, S.L.S., Godoy, P.B. and Muellar-Harvey, I. (2005) Do all tannins have similar nutritional effects? A comparison of three Brazilian fodder legumes. Animal Feed Science and Technology, 119, 345- 361. doi:10.1016/j.anifeedsci.2004.06.004
- Silanikove, N., Shinder, D., Gilboa, N., Eyal, M. and Nitsan, Z. (1996) Binding of polyethylene glycol to samples of forage plants as an assay of tannins and their negative effects on ruminal degradation. Journal of Agricultural and Food Chemistry, 44, 3230-3234. doi:10.1021/jf9602277
- Haddi, M.L., Filacorda, S., Miniai, K., Rollin, F. and Susmel, P. (2003) In vitro fermentation kinetics of some halophyte shrubs sampled at three stages of maturity. Animal Feed Science and Technology, 104, 215-225. doi:10.1016/S0377-8401(02)00323-1
- Osuga, I.M., Abdulrazak, S.A., Ichinoe, T. and Fujihara, T. (2005) Chemical composition, degradation characteristics and effect of tannin on digestibility of some browse species from Kenya harvested during the wet season. Asian Australian Journal of Animal Science, 1, 54-60.
- Fievez, V., Babayemi, O.J. and Demeyer, D. (2005) Estimation of direct and indirect gas production in syringes: A tool to estimate short chain fatty acid production that requires minimal laboratory facilities. Animal Feed Science and Technology, 123-124, 197-210. doi:10.1016/j.anifeedsci.2005.05.001
- Getachew, G., Makkar, H.P.S. and Becker, K. (2000) Effect of polyethylene glycol on in vitro degradability of nitrogen and microbial protein synthesis from tannin-rich browse and herbaceous legumes. British Journal of Nutrition, 84, 73-83.
- Waghorn, G.C., Shelton, I.D. and McNabb, W.C. (1994) Effect of CT in Lotus pedunculatus on its nutritive value for sheep 1: Non-nitrogenous aspects. Journal of Agricultural Science Cambridge, 123, 99-107. doi:10.1017/S0021859600067824
- Madibela, O.R., Seitshiro, O. and Mochankana, M.E. (2006) Deactivation effects of polyethylene glycol (PEG) on in vitro dry matter digestibility of Colophospermum mopane (Mophane) and Acacia browse trees in Botswana. Pakistan Journal of Nutrition, 5, 343-347. doi:10.3923/pjn.2006.343.347
- Makkar, H.P.S. and Becker, K. (1996) A bioassay for tannins. Polyphenols Communications, 96, Proceedings of XVIIIth International Conference on Polyphenols, Bordeaux, 15-18 July 1996, 197-198.

