Journal of Cancer Therapy
Vol.4 No.2(2013), Article ID:30001,9 pages DOI:10.4236/jct.2013.42077
Chemosensitivity Testing of Circulating Epithelial Tumor Cells (CETC) in Vitro: Correlation to in Vivo Sensitivity and Clinical Outcome
![]()
1Clinic for Internal Medicine II, University Hospital, Friedrich Schiller University, Jena, Germany; 2Transfusionsmedizinisches Zentrum, Bayreuth, Germany; 3Onkologische Schwerpunktpraxis, Kronach, Germany; 4Women’s Hospital, University Hospital, Friedrich Schiller University, Jena, Germany.
Email: *kpachmann@laborpachmann.de
Copyright © 2013 Nadine Rüdiger et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 25th, 2013; revised March 26th, 2013; accepted April 2nd, 2013
Keywords: Circulating Epithelial Tumor Cells; Chemosensitivity Testing; Breast Cancer; Ovarian Cancer
ABSTRACT
Background: Chemotherapy is a mainstay of tumor therapy, however, it is predominantly applied according to empirically developed recommendations derived from statistical relapse rates occurring years after the treatment in the adjuvant situation and from progression-free interval data in the metastatic situation, without any possibility of individually determining the efficacy in the adjuvant situation and with loss of time and quality of life in the metastatic situation if the drugs chosen are not effective. Here, we present a method to determine the efficiency of chemotherapeutic drugs using tumor cells circulating in blood as the part of the tumor actually available in the patient’s body for chemosensitivity testing. Methodology/Principal Findings: After only red blood cell lysis, omitting any enrichment (analogous to other blood cell enumeration methods, including rare CD34 cells), the white cells comprising the circulating epithelial tumor cells (CETC) are exposed to the drugs in question in different concentrations and for different periods of time. Staining with a fluorescence-labeled anti-epithelial antibody detects both vital and dying tumor cells, distinguishing vital from dying cells through membrane permeability and nuclear staining with propidium iodide. Increasing percentages of dying tumor cells are observed dependent on time and concentration. The sensitivity can vary during therapy and was correlated with decrease or increase in CETC and clinical outcome. Conclusions/Significance: Thus, we are able to show that chemosensitivity testing of circulating tumor cells provides real-time information about the sensitivity of the tumor present in the patient, even at different times during therapy, and correlates with treatment success.
1. Introduction
For patients diagnosed with a malignant tumor, cure is presumably only possible if the tumor is completely eradicated. Initially, the main aim is to eliminate the primary tumor, the major tumor burden, preferentially by surgery. However, most cancer patients do not die from their primary tumor but from distant metastases, developing some years after the removal of the primary tumor. During tumor growth, cells from the tumor are disseminated continuously via lymph vessels or directly into blood [1]. These cells are assumed to be the source of metastasis formation. Patients with affected lymph nodes have a less favorable chance of disease-free survival than patients without lymph-node-positive disease, indicating that cells detached from the tumor were able to settle and grow in foreign tissue. Therefore, as the second pillar of tumor therapy, chemotherapy has evolved and is applied after surgery as adjuvant chemotherapy, e.g. in breast and ovarian cancer, to eliminate such early disseminated cells, when no detectable tumor is present. Such therapies have been shown to avert metastasis formation and ultimately save lives in breast cancer patients [2]. In the adjuvant situation, these therapies have been developed in clinical trials using the statistical improvement of relapse-free survival as a measure. This cannot, however, predict for the individual patient whether the chosen treatment will be successful in his/her case. Furthermore, if metastases develop, even systemic therapies can rarely cure the patient. It would, therefore, be of great value to be able to determine the effectiveness of chemotherapies in the individual patient prior to starting the treatment.
For this purpose, a variety of approaches has been developed to test the effectiveness of different agents beforehand.
Some of these tests require culturing systems of cell lines [3-7] or relying on culturing tumor material from the primary tumor [8-11]. In many situations, however, material from the primary tumor is no longer available. In addition, tumors consist of heterogeneous cell populations [12,13] which may respond differently in culture [14], and it is not clear which cell subpopulation will finally be able to form metastases. A more recent approach is testing chemosensitivity of the tumor based on its shrinkage during neoadjuvant chemotherapy, especially in breast cancer [15,16]. Outcomes seem to be more favorable in patients in whom viable tumor cells are no longer detectable (pCR) and patients with residual tumors may have an unfavorable outcome [17]. Due to the particularly poor outcome of patients with HER2/neupositive breast cancer or patients with triple-negative breast cancer not reaching pCR [18], this treatment is not an option to determine chemosensitivity in patients with these types of tumors as long as no markers are available to ensure that pathological complete response will be achieved [19,20]. In addition, we have shown previously that tumor shrinkage may, in part, be due to the release of tumor cells which may later be responsible for a relapse [21].
Several methods to detect tumor cells in the peripheral blood are available, which use enrichment procedures due to the assumed rarity of such cells. The accuracy of all of these methods is affected by the at times massive cell loss, preventing correct enumeration [22].
In contrast, all blood cell enumeration methods, including methods for rare cell enumeration like CD34-positive stem cells, avoid preanalytic manipulation of the samples [23].
Using a comparable low-loss approach with only red blood cell lysis and one centrifugation step also for circulating tumor cell enumeration, we have shown previously that all tumor cells spiked into normal blood can be retrieved [24] and that considerably more cells are retrieved from patients’ blood than e.g. with the CellSearchTM approach [25]. Using this non-dissipative approach for circulating epithelial cell detection and enumeration for monitoring treatment success by repeated analyses, increasing numbers of CETC in breast cancer patients during or after chemoand/or maintenance therapy [26-29] indicate increased tumor activity with an increased probability of relapse. These CETC could be used to test drug sensitivity not only in the metastatatic but as early as in the adjuvant situation. In metastatic disease, where it is often difficult to choose between different therapy options, valuable time could be gained if it were possible to determine in advance whether the tumor cells will respond to a particular treatment.
Loss of membrane integrity is one of the earliest indicators of cell damage and a sign of serious injury. It leads to cell death [30] most probably due to changes in the permeability and fluidity of the cell membrane as well as changes in cellular uptake and intracellular metabolism, shown to occur within hours [31]. In the present report we provide evidence that in vitro chemosensitivity testing of CETC using propidium iodide uptake, which results in nuclear red fluorescence, allows assessment, before treatment, of whether the tumor cells will be sensitive to the intended drugs. This test can performed repeatedly and we provide data showing that the sensitivity of CETC to the respective drug in vitro correlates with in vivo response. Lack of sensitivity is predictive of poor outcome, whereas a high sensitivity is a predictor of good clinical outcome.
2. Patients and Methods
Cells from peripheral blood from cancer patients were prepared according to the published method [24], using only red blood cell lysis and one centrifugation step. In short, 1 ml of blood was mixed with 10 ml of lysis buffer (Quiagen) for ten minutes in the cold and spun at 700 g for ten minutes at room temperature. Epithelial cells were detected using EpCAM (CD326), an anti-epithelial antibody, conjugated with fluorescein isothionate (FITC). Dying cells were detected using propidium iodide (PI) which only stains the nuclei of dying cells with a permeable membrane. The cell suspension was subsequently incubated under cell culture conditions with the drugs in question. Cell kill effectiveness was determined by exposing all white cells from the cell pellet of 100 µl of blood to different concentrations of the respective agent in medium, where the concentration calculated to be present in the blood of patients under treatment was set as one and a tenfold lower and a tenfold higher concentration was tested in comparison to the control suspendsion containing the cells without addition of the agent. Cells were short-time cultured under these conditions for up to nine hours and the cytotoxic effect measured at three, six and nine hours. Cells could be characterized as follows: 1) Live blood cells appearing only in transmitted light with no fluorescence staining; 2) Dead blood cells where the membrane had become permeable showing propidium iodide entering the cell, staining the nucleus red fluorescent; 3) Live epithelial (presumably tumor) cells staining with green fluorescence, preferentially as a cap, due to reactivity with FITC-conjugated anti-EpCAM; 4) Dead CETC with simultaneous green and red fluorescent staining, resulting either in a clear green cap with a red nucleus, or later during nucleic and cell disintegration with an orange combination stain. The numbers of live and dead CETC were determined at each point in time without and with the indicated concentrations of the therapeutic agent and the percentage increase in dead cells over the control was calculated. Quantitative analysis of the samples at different times after incubation with the respective drugs was performed using the image analysis system of the ScanR (Olympus Hamburg, Germany), allowing repeated scanning of the same area. A typical example, showing typical live and dead cells, is shown in Figure 1.
3. Results
Patients with breast and ovarian cancer were tested for the chemosensitivity of tumor cells among the white blood cells to frequently used chemotherapeutic agents. The drug concentrations used were calculated to be equivalent to the therapeutic concentrations, assuming a dilution in five liters of blood. All white blood cells from 1 ml of blood containing the tumor cells were incubated with the respective drug concentrations. Tumor cells were distinguished from remnant blood cells by their staining with fluorochrome-labeled anti-EpCAM. EpCAM-positive cells retrieved among the unseparated white blood cells from a final volume of 100 µl of blood, including cells with very low EpCAM expression (see

Figure 1. Gallery of live epithelial antigen-positive cells with typical green fluorescent cap-like staining with FITC-antiCD326 and of dead epithelial antigen-positive cells with the green fluorescent caps and additional nuclear red fluorescent staining with propidium iodide.
tiny spots in Figure 1), presumably cells in epithelial/ mesenchymal transition, were counted and the proportion of live and dead cells determined as described in the methods section. During incubation, increases in the percentages of propidium iodide-positive dying cells compared to the samples from the same patients without the drug were noted, resulting from the killing of the sensitive cells. Typical dose-response curves of the CETC of two breast cancer patients to the drug doxorubicin after six hours of short-term culture are shown in Figure 2.
In triplicate assays with increasing drug concentrations, 70% cell kill was achieved at the concentration equivalent to the therapeutic concentration with cells of a breast cancer patient sensitive to the drug, and less than 10% with cells of another breast cancer patient whose cells were resistant to the drug. The respective standard deviations show that the results are highly reproducible. In Figure 3, typical dose-response curves of the cells of another breast cancer patient for two different drugs, doxorubicin and capecitabine, are shown after three, six and nine hours, to ensure that late effects are captured as well.
Up to 90% cell kill was achieved with the highly effective drug doxorubicin (Figure 3(a)) and 30% - 40% with the marginally effective drug capecitabine (Figure 3(b)). Effects were highly reproducible after three and six hours. Since percentages are referred to the control sample without the drug the effect was less obvious in the nine-hour sample due to increased cell decay at that point in time; therefore, the six-hour incubation period was preferred in subsequent analyses.
In ovarian cancer, the need for tumor cell sensitivity testing is even more pressing, since so far the outcomes achieved have been unsatisfactory. In 39 patients with ovarian cancer the response of the tumor cells circulating in the blood to the two agents, carboplatin and taxane,
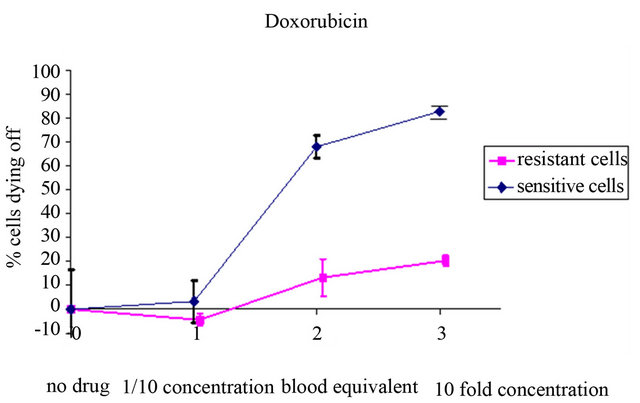
Figure 2. Concentration-dependent increase in the percentage of dying CETC during incubation with different concentrations in a patient with cells sensitive to and a patient with cells resistant to the drug doxorubicin.

Figure 3. (a) and (b) Typical time and concentration-dependent increase in the percentage of dying CETC from a breast cancer patient during incubation with different concentrations of (a) a highly effective drug (b) a marginally effective drug.
used according to the guidelines for first line chemotherapy, was analyzed. In Figures 4(a) and (b), typical results for two patients with ovarian cancer are shown.
The first patient with a tumor classified as pT3c L0 V0 pN1 G3 R0 received six cycles of carboplatin and paclitaxel after surgery. Her CETC counts dropped more than thirty-fold and she is well and has been relapsed-free now for two years after diagnosis. The sensitivity of her CETC to both agents was more than 50% at both instances of testing, even at the end of chemotherapy. The second patient with a comparable tumor (pT3c L2 V0 pN1 G3 R0) also received six cycles of carboplatin and paclitaxel after surgery. Her CETC count dropped initially, but then started to increase again almost immediately. The sensitivity of her CETC to both agents at that time was below 50% and she experienced a relapse 1.3 years after diagnosis.
A threshold of sensitivity for patients’ cells was set and cells which showed less than 50% response to carboplatin and paclitaxel were classified as resistant. The progression-free survival of the 39 patients was analyzed, comparing patients resistant to the two agents with those sensitive to the drugs. Most tumors were FIGO III (50% in patients with resistant cell populations and 50% in patients with sensitive cell populations) and FIGO IV (59% in the resistant and 41% in the sensitive group respectively) (Table 1).
There were more patients with involved lymph nodes in the resistant group than in the sensitive group (67% vs. 43%); however, this difference was not statistically significant (p = 0.14). All patients were locally macroscopically tumor-free after surgery. The Kaplan-Meier progression-free survival curves are shown in Figure 4. Patients with resistant cells had a significantly shorter progression-free survival (p = 0.007, hazard ratio = 0.30, 95% confidence interval: 0.1217 - 0.7519) than patients with sensitive cells in the in vitro testing (Figure 5).
An example showing how chemosensitivity testing might help to tailor the chemotherapy to the requirements of an individual patient’s cells is shown in Figure 6 in a patient with an ovarian carcinoma FIGO IV.
The number of CETC increased continuously despite six cycles of therapy with carboplatin and paclitaxel, while the patient experienced disease progression. At both instances of testing during therapy, the chemosensitivity of her CETC to carboplatin was approximately 50% and to paclitaxel below 50%. After the end of chemotherapy, sensitivity to carboplatin recovered, but to paclitaxel remained very low. In contrast, sensitivity to anthracycline was high at all testing times and remained high. Due to progressive disease, the patient received lyposomal doxorubicin. Under this treatment, her CETC instantly declined and her liver metastases remained stable. However, after the end of salvage therapy metastases started growing again and a third-line therapy with topotecan was initiated.
Thus, drug sensitivity is not necessarily a stable property of these cells and can change during therapy, as also shown in Figure 7 for a breast cancer patient treated with an adjuvant chemotherapy schedule comprising an anthracycline, a taxane and cyclophosphamide.
Even though the patient received the three drugs sequentially, chemosensitivity declined for all three drugs simultaneously. At the time of the lowest efficacy, the number of CETC started rising again. The patient is now under continuous treatment with hormone blocking therapy which led again to the elimination of the CETC and she is relapse-free.
Table 1. Distribution of tumor stages and resistance and sensitivity to the drug combination carboplatin/paclitaxel among 39 patients with ovarian cancer (res = resistant, sens = sensitive).


Figure 4. (a) (b) Constantly high chemosensitivity of the CETC of a patient with ovarian cancer to the two applied drugs with declining numbers of CETC during treatment and CR during the observation time (Carbo = Carboplatin, Pac = Paclitaxel); (b) Low chemosensitivity of the CETC of a patient with ovarian cancer to the two applied drugs with re-increasing numbers of CETC during treatment and early relapse.


Figure 5. Kaplan-Meyer progression-free survival curves of patients sensitive to (green line) and resistant to (red line) the drugs applied according to the guidelines, showing a highly significant difference.

Figure 6. Low and changing chemosensitivity of the CETC of a patient with ovarian cancer to the two initially applied drugs with increasing numbers of CETC during this treatment and progressive disease with a constantly high sensitivity to the third drug, doxorubicin. After exposure to this drug, cell numbers declined and a progression-free interval was obtained. Carbo = Carboplatin, Pac = Paclitaxel, Doxo = Doxorubicin.
In a patient with metastasized breast cancer, a direct comparison of the chemosensitivity to doxorubicin of CETC and cells of an index metastasis, the size of which was carefully determined before and after treatment, was performed (Figure 8).
In spite of therapy with vinorelbine and trastuzumab, the patient experienced progressive disease along with increasing numbers of CETC indicating resistance to

Figure 7. Changing chemosensitivity of CETC of a breast cancer patient to three drugs applied sequentially.

Figure 8. Concomitant increase in the CETC and an index metastasis during initial ineffective treatment, but subsequent decrease in the CETC numbers as well as the size of the index metastasis after changing to the drug that had been shown to be effective in chemosensitivity testing. Tramb = Trastuzumab.
these drugs. However, the patient’s cells showed a high sensitivity to anthracycline and the patient was subsequently treated with a combination of anthracycline and taxane. A good reduction of the CETC was seen over time, accompanied by a reduction in the size of the index metastasis.
4. Discussion
So far, chemosensitivity testing has only been performed in cell cultures derived from primary tumors or cell lines [30,31], using methods such as proliferation assays and in vitro clonogenic assays, microfluidic approaches and multidrug resistance assays [32-36], cell metabolic activity assays [37-39], molecular assays to monitor expression of markers for responsiveness [40], and in vivo imaging assays [41]. These approaches, however, all have major drawbacks. The results from cell lines [3, 42] may not be comparable to those from real tumor cells.
Cell culturing may alter the properties of cells derived from the primary tumor [35,41] and, most importantly, chemotherapy is mainly directed against the remnant cells in the body after removal of the primary tumor and these cells may only in part carry the characteristics of the cells from the primary tumor [9,36,43], especially after repeated therapies. Interpretation of proliferation assays as well as metabolic assays requires samples consisting of a uniform population of cells, which is often not achievable.
Circulating epithelial cells, most probably cells released from the tumor, which can be detected in almost all cancer patients using our nondissipative approach [24], have been shown to respond to therapy in the same way as the primary tumor [1] and, therefore, it seems appropriate to test the actual sensitivity of the residual tumor burden to chemotherapeutic agents.
Circulating tumor cells can be distinguished from the remnant blood cells by the expression of the surface molecule EpCAM. Approaches using this surface antigen for cell enrichment require a sufficiently high expression of the molecule to be able to capture these cells, which may lead to massive losses of the respective cells [22], especially among those with low expression, such as cells in epithelial/mesenchymal transition, presumably cancer stem cells. In contrast, straightforward determination of all positive cells without enrichment steps using detection with a fluorescent antibody and image analysis avoids all bias introduced by additional manipulation. It should be emphasized that, apart from proving the presence of the same mutation in the primary tumor and in the circulating tumor cells, which is to date not possible in breast and ovarian cancer, none of these methods can unequivocally prove the affiliation of the cells to the tumor. Still, we have shown in cancer patients that the cells detected by us reflect the response of the tumor to therapy [1]. The first step occurring as a result of the cytotoxic effect of chemotherapeutic agents is increased cell permeability. Additional staining of the epithelial antigen-positive cells with a fluorescent dye, which can enter the cell only if membrane integrity is lost and shows increased fluorescence when it intercalates with the DNA, allows testing the epithelial cells selectively for membrane integrity during short term incubation with the respective drugs, even in the presence of other cells. The feasibility of this approach is demonstrated in the present report.
We could show that CETC increasingly undergo cell death with time and in relation to drug concentration. In breast cancer patients, doxorubicin, an agent with proven effectiveness, was also shown to be the most effective drug in in vitro chemosensitivity testing.
A typical example of the predictive relevance of chemosensitivity testing for clinical outcome is the differential response to treatment observed in two ovarian cancer patients, one of whom was sensitive to carboplatin and paclitaxel and had decreasing CETC numbers and has been relapse-free until the last visit. The other patient with low sensitivity of the CETC to these agents showed increasing cell numbers during therapy and experienced early relapse. Among ovarian cancer patients in whom these cells in the peripheral blood were tested for sensitivity to these drugs used according to the guidelines, the circulating cells did indeed respond or were resistant to the same degree in vivo as in vitro and this was highly relevant for relapse-free survival, too.
In addition, here we show that in vitro chemosensitivity changes that take place during therapy, a well known phenomenon in breast and ovarian cancer [44,45], may be relevant for clinical outcome. An ovarian cancer patient showing increasing CETC numbers during therapy along with low and decreasing sensitivity, especially to taxane, and progressive disease, was still sensitive to doxorubicin and responded to subsequent treatment with liposomal doxorubicin.
In a case of metastatic breast cancer, cells responded well in vitro, CETC numbers in the blood gradually declined to below detection threshold and an index metastatic lesion shrank in size.
Thus, here we present for the first time an approach whereby the true targets of systemic chemotherapy, the tumor cells remaining in the body after surgery, can be tested in individual patients for their response to different chemotherapeutic drugs. This will help to develop effective cancer therapies, allow individually targeted therapies, and spare patients unnecessary treatments; indeed, it has the potential to contribute to future cost savings in the healthcare system.
REFERENCES
- O. Camara, M. Rengsberger, A. Egbe, A. Koch, M. Gajda, U. Hammer, C. Jörke, C. Rabenstein, M. Untch and K. Pachmann, “The Relevance of Circulating Epithelial Tumour Cells (CETC) for Therapy Monitoring during Neoadjuvant (Primary Systemic) Chemotherapy in Breast Cancer,” Annals of Oncology, Vol. 18, No. 9, 2007, pp. 1484- 1492. doi:10.1093/annonc/mdm206
- G. Bonadonna, A. Moliterni, M. Zambetti, M. G. Daidone, S. Pilotti, L. Gianni and P. Valagussa, “30 Years’ Follow Up of Randomised Studies of Adjuvant CMF in Operable Breast Cancer: Cohort Study,” British Medical Journal, Vol. 330, No. 7485, 2005, p. 217. doi:10.1136/bmj.38314.622095.8F
- E. Colomb and P. M. Martin, “Testing of a Chemosensitivity Screening Method on Sensitive and Resistant Breast Tumoral Epithelial Cell Lines,” Analytical Cellular Pathology, Vol. 6, No. 2, 1994, pp. 105-116.
- F. X. Han, H. Lin and L. Ru, “MTT Assay for Detecting 5-Fluorouracil Chemosensitivity of Human Breast Carcinoma Cell Line,” Journal of Southern Medical University, Vol. 29, No. 1, 2009, pp. 97-99.
- T. P. Dawson, R. V. Iyer, R. W. Lea, P. Roberts, E. Harris, K. Ashton, A. Golash and C. H. Davis, “The MTS vs. the ATP Assay for in Vitro Chemosensitivity Testing of Primary Glioma Tumour Culture,” Neuropathology and Applied Neurobiology, Vol. 36, No. 6, 2010, pp. 564-567. doi:10.1111/j.1365-2990.2010.01096.x
- K. Brigulová, M. Cervinka, J. Tosner and I. Sedláková, “Chemoresistance Testing of Human Ovarian Cancer Cells and Its in Vitro Model,” Toxicology in Vitro, Vol. 24, No. 8, 2010, pp. 2108-2115. doi:10.1016/j.tiv.2010.08.010
- P. J. Schuler, S. Trellakis, J. Greve, M. Bas, C. Bergmann, E. Bölke, G. Lehnerdt, S. Mattheis, A. E. Albers, S. Brandau, S. Lang, T. L. Whiteside, H. Bier and T. K. Hoffmann, “In Vitro Chemosensitivity of Head and Neck Cancer Cell Lines,” European Journal of Medical Research, Vol. 15, No. 8, 2010, pp. 337-344. doi:10.1186/2047-783X-15-8-337
- A. M. Otto, M. Brischwein, H. Grothe, E. Motrescu and B. Wolf, “Multiparametric Sensor Chips for Chemosensitivity Testing of Sensitive and Resistant Tumor Cells,” Recent Results in Cancer Research, Vol. 161, 2003, pp. 39-47. doi:10.1007/978-3-642-19022-3_4
- S. Ugurel, D. Schadendorf, C. Pföhler, K. Neuber, A. Thoelke, J. Ulrich, A. Hauschild, K. Spieth, M. Kaatz, W. Rittgen, S. Delorme, W. Tilgen, U. Reinhold and Dermatologic Cooperative Oncology Group, “In Vitro Drug Sensitivity Predicts Response and Survival after Individualized Sensitivity-Directed Chemotherapy in Metastatic Melanoma: A Multicenter Phase II Trial of the Dermatologic Cooperative Oncology Group,” Clinical Cancer Research, 2006, Vol. 12, No. 18, pp. 5454-5463. doi:10.1158/1078-0432.CCR-05-2763
- T. Wakatsuki, A. Irisawa, H. Imamura, M. Terashima, G. Shibukawa, T. Takagi, Y. Takahashi, A. Sato, M. Sato, T. Ikeda, R. Suzuki, T. Hikichi, K. Obara and H. Ohira, “Complete Response of Anaplastic Pancreatic Carcinoma to Paclitaxel Treatment Selected by Chemosensitivity Testing,” International Journal of Clinical Oncology, Vol. 15, No. 3, 2010, pp. 310-313. doi:10.1007/s10147-010-0038-9
- T. Wakatsuki, A. Irisawa, M. Terashima, G. Shibukawa, T. Takagi, H. Imamura, Y. Takahashi, A. Sato, M. Sato, T. Ikeda, R. Suzuki, T. Hikichi, K. Obara and H. Ohira, “ATP Assay-Guided Chemosensitivity Testing for Gemcitabine with Biopsy Specimens Obtained from unresectable Pancreatic Cancer Using Endoscopic Ultrasonography-Guided Fine-Needle Aspiration,” International Journal of Clinical Oncology, Vol. 16, No. 4, 2011, pp. 387- 394. doi:10.1007/s10147-011-0197-3
- B. U. Sevin and J. P. Perras, “Tumor Heterogeneity and in Vitro Chemosensitivity Testing in Ovarian Cancer,” American Journal of Obstetrics and Gynecology, Vol. 176, No. 4, 1997, pp. 759-768. doi:10.1016/S0002-9378(97)70599-4
- T. J. Herzog, T. C. Krivak, A. N. Fader and R. L. Coleman, “Chemosensitivity Testing with ChemoFx and Over- all Survival in Primary Ovarian Cancer,” American Journal of Obstetrics and Gynecology, Vol. 203, No. 1, 2010, pp. 68.e1-6.
- Z. Q. Ling, C. J. Qi, X. X. Lu, L. J. Qian, L. H. Gu, Z. G. Zheng, Q. Zhao, S. Wang, X. H. Fang, Z. X. Yang, J. Yin and W. M. Mao, “Heterogeneity of Chemosensitivity in Esophageal Cancer Using ATP-Tumor Chemosensitivity Assay,” Acta Pharmacolpgoca Sinica, Vol. 33, No. 3, 2010, pp. 401-406. doi:10.1038/aps.2011.195
- I. Vergote, C. G. Tropé, F. Amant, G. B. Kristensen, T. Ehlen, N. Johnson, R. H. Verheijen, M. E. van der Burg, A. J. Lacave, P. B. Panici, G. G. Kenter, A. Casado, C. Mendiola, C. Coens, L. Verleye, G. C. Stuart, S. Pecorelli, N. S. Reed, European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group and NCIC Clinical Trials Group, “Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer,” New England Journal of Medicine, Vol. 363, No. 10, 2010, pp. 943-953. doi:10.1056/NEJMoa0908806
- J. Aigner, A. Schneeweiss, C. Sohn and F. Marmé, “The Role of Neoadjuvant Chemotherapy in the Management of Primary Breast Cancer,” Minerva Ginecologica, Vol. 63, No. 3, 2011, pp. 261-274.
- M. Untch, P. A. Fasching, G. E. Konecny, S. Hasmüller, A. Lebeau, R. Kreienberg, O. Camara, V. Müller, A. du Bois, T. Kühn, E. Stickeler, N. Harbeck, C. Höss, S. Kahlert, T. Beck, W. Fett, K. M. Mehta, G. von Minckwitz and S. Loibl, “Complete Response after Neoadjuvant Chemo-Therapy plus Trastuzumab Predicts Favourable Survival in Human Epidermal Growth Factor Receptor 2- Overexpressing Breast Cancer: Results from the TECHNO Trial of the AGO and GBG Study Groups,” Journal of Clinical Oncology, 2011, Vol. 29, No. 25, pp. 3351-3357. doi:10.1200/JCO.2010.31.4930
- C. Liedtke, C. Mazouni, K. R. Hess, F. André, A. Tordai, J. A. Mejia, W. F. Symmans, A. M. Gonzalez-Angulo, B. Hennessy, M. Green, M. Cristofanilli, G. N. Hortobagyi and L. Pusztai, “Response to Neoadjuvant Therapy and LongTerm Survival in Patients with Triple-Negative Breast Cancer,” Journal of Clinical Oncology, Vol. 26, No. 8, 2008, pp. 1275-1281. doi:10.1200/JCO.2007.14.4147
- C. Shimizu, N. Masuda, K. Yoshimura, H. Tsuda, M. Mano, M. Ando, K. Tamura and Y. Fujiwara, “LongTerm Outcome and Pattern of Relapse after Neoadjuvant Chemotherapy in Patients with Human Epidermal Growth Factor Receptor 2-Positive Primary Breast Cancer,” Japanese Journal of Clinical Oncology, Vol. 39, No. 8, 2009, pp. 484-490. doi:10.1093/jjco/hyp052
- T. Karn, L. Pusztai, U. Holtrich, T. Iwamoto, C. Y. Shiang, M. Schmidt, V. Müller, C. Solbach, R. Gaetje, L. Hanker, A. Ahr, C. Liedtke, E. Ruckhäberle, M. Kaufmann and A. Rody, “Homogeneous Datasets of Triple Negative Breast Cancers Enable the Identification of Novel Prognostic and Predictive Signatures,” PLoS One, Vol. 6, No. 12, 2012, Article ID: e28403. doi:10.1371/journal.pone.0028403
- M. Gajda, O. Camara, S. Oppel, T. Kroll, C. Jörke, S. Krauspe, U. Hammer, C. Rabenstein, M. Untch, I. B. Runnebaum and K. Pachmann, “Monitoring Circulating Epithelial Tumor Cells (CETC) during Primary Systemic Chemotherapy Including Trastuzumab for Early Prediction of Outcome in Patients with Her2/neu-Positive Tumors,” Annals of Oncology, Vol. 19, No. 12, 2008, pp. 2090-2091. doi:10.1093/annonc/mdn648
- Z. Panteleakou, P. Lembessis, A. Sourla, N. Pissimissis, A. Polyzos, C. Deliveliotis and M. Koutsilieris, “Detection of Circulating Tumor Cells in Prostate Cancer Patients: Methodological Pitfalls and Clinical Relevance,” Molecular Medicine, Vol. 15, No. 3-4, 2009, pp. 101-114.
- S. Mortazavi, F. A. Ardalan, S. R. Nodehi, F. F. Karder and N. Miraliakbari, “True Volumetric Method for Flow Cytometric Enumeration of CD34 + Stem Cells and Its Agreement with a Standard Bead-Based Single-Platform Protocol,” Cytotherapy, Vol. 14, No. 5, 2012, pp. 621- 629. doi:10.3109/14653249.2012.667875
- K. Pachmann, J. H. Clement, C. P. Schneider, B. Willen, O. Camara, U. Pachmann and K. Höffken, “Standardized Quantification of Circulating Peripheral Tumor Cells from Lung and Breast Cancer,” Clinical Chemistry and Laboratory Medicine, Vol. 43, No. 6, 2005, pp. 617-627. doi:10.1515/CCLM.2005.107
- U. Pachmann, K. Hekimian, S. Carl, N. Ruediger, C. Rabenstein and K. Pachmann, “Comparing Sequential Steps for Detection of Circulating Tumor Cells: More Specific or Just Less Sensitive?” WebmedCentral CANCER, Vol. 2, No. 2, 2011, Article ID: WMC001490.
- K. Pachmann, O. Camara, A. Kavallaris, S. Krauspe, N. Malarski, M. Gajda, T. Kroll, C. Jörke, U. Hammer, A. Altendorf-Hofmann, C. Rabenstein, U. Pachmann, I. Runnebaum and K. Höffken, “Monitoring the Response of Circulating Epithelial Tumor Cells (CETC) to Adjuvant Chemotherapy in Breast Cancer Allows Detection of Patients at Risk of Early Relapse,” Journal of Clinical Oncology, Vol. 26, No. 6, 2008, pp. 1208-1215. doi:10.1200/JCO.2007.13.6523
- O. Camara, M. Rengsberger, A. Egbe, A. Koch, M. Gajda, U. Hammer, C. Jörke, C. Rabenstein, M. Untch and K. Pachmann, “The Relevance of Circulating Epithelial Tumour Cells (CETC) for Therapy Monitoring during Neoadjuvant (Primary Systemic) Chemotherapy in Breast Cancer,” Annals of Oncology, Vol. 18, No. 9, 2007, pp. 1484-1492. doi:10.1093/annonc/mdm206
- K. Pachmann, O. Camara, A. Kohlhase, C. Rabenstein, T. Kroll, I. B. Runnebaum and K. Hoeffken, “Assessing the Efficacy of Targeted Therapy Using Circulating Epithelial Tumor Cells (CETC): The Example of SERM Therapy Monitoring as a Unique Tool to Individualize Therapy,” Journal of Cancer Research and Clinical Oncology, Vol. 137, No. 5, 2011, pp. 821-828. doi:10.1007/s00432-010-0942-4
- K. Pachmann, O. Camara, T. Kroll, M. Gajda, A. K. Gellner, J. Wotschadlo and I. B. Runnebaum, “Efficacy Control of Therapy Using Circulating Epithelial Tumor Cells (CETC) as ‘Liquid Biopsy’: Trastuzumab in HER2/neuPositive Breast Carcinoma,” Journal of Cancer Research and Clinical Oncology, Vol. 137, No. 9, 2011, pp. 1317- 1327. doi:10.1007/s00432-011-1000-6
- J. C. Davila, C. G. Reddy, P. J. Davis and D. Acosta, “Toxicity Assessment of Papaverine Hydrochloride and Papaverine-Derived Metabolites in Primary Cultures of Rat Hepatocytes,” In Vitro Cellular and Developmental Biology, Vol. 26, No. 5, 1990, pp. 515-524. doi:10.1007/BF02624095
- N. Rasmussen, J. H. Andersen, H. Jespersena, O. G. Mouritsen and H. J. Ditzel, “Effect of Free Fatty Acids and Lysolipids on Cellular Uptake of Doxorubicin in Human Breast Cancer Cell Lines,” Anti-Cancer Drugs, Vol. 21, No. 7, 2010, pp. 674-677.
- V. K. Sondak, C. A. Bertelsen, N. Tanigawa, S. U. Hildebrand-Zanki, D. L. Morton, E. L. Korn and D. H. Kern, “Clinical Correlations with Chemosensitivities Measured in a Rapid Thymidine Incorporation Assay,” Cancer Research, Vol. 44, No. 4, 1984, pp. 1725-1728.
- H. Eidtmann, W. Jonat and H. Maass, “Drug Sensitivity Testing of Gynecologic Tumors Using Volm’s Test and Stem Cell Assay,” Geburtshilfe und Frauenheilkunde, Vol. 45, No. 7, 1985, pp. 477-481. doi:10.1055/s-2008-1036356
- J. M. Lyons 3rd, J. Abergel, J. L. Thomson, C. T. Anthony, Y. Z. Wang, L. B. Anthony, J. P. Boudreaux, J. Strauchen, M. Idrees, R. R. Warner and E. A. Woltering, “In Vitro Chemoresistance Testing in Well-Differentiated Carcinoid Tumors,” Annals of Surgical Oncology, Vol. 16, No. 3, 2009, pp. 649-655. doi:10.1245/s10434-008-0261-z
- J. Komen, F. Wolbers, H. R. Franke, H. Andersson, I. Vermes and A. van den Berg, “Viability Analysis and Apoptosis Induction of Breast Cancer Cells in a Microfluidic Device: Effect of Cytostatic Drugs,” Biomedical Microdevices, Vol. 10, No. 5, 2008, pp. 727-737. doi:10.1007/s10544-008-9184-5
- Z. Li, H. P. Song, W. S. He, Y. Tian and T. Huang, “In Vitro Chemosensitivity Testing of Primary and Recurrent Breast Carcinomas and Its Clinical Significance,” Journal of Huazhong University of Science and Technology, Vol. 28, No. 6, 2008, pp. 683-687. doi:10.1007/s11596-008-0616-5
- A. M. Otto, M. Brischwein, A. Niendorf, T. Henning, E. Motrescu and B. Wolf, “Microphysiological Testing for Chemosensitivity of Living Tumor Cells with Multiparametric Microsensor Chips,” Cancer Detection and Prevention, Vol. 27, No. 4, 2003, pp. 291-296. doi:10.1016/S0361-090X(03)00093-X
- C. M. Kurbacher and I. A. Cree, “Chemosensitivity Testing Using Microplate Adenosine Triphosphate-Based Luminescence Measurements,” Methods in Molecular Medicine, Vol. 110, 2005, pp. 101-120.
- F. X. Han, H. Lin and L. Ru, “MTT Assay for Detecting 5-Fluorouracil Chemosensitivity of Human Breast Carcinoma Cell Line,” Journal of Southern Medical University, Vol. 29, No. 1, 2009, pp. 97-99.
- P. Gazzaniga, G. Naso, A. Gradilone, E. Cortesi, O. Gandini, W. Gianni, M. A. Fabbri, B. Vincenzi, F. di Silverio, L. Frati, A. M. Aglianò and M. Cristofanilli, “Chemosensitivity Profile Assay of Circulating Cancer Cells: Prognostic and Predictive Value in Epithelial Tumors,” International Journal of Cancer, Vol. 126, No. 10, 2010, pp. 2437-2447.
- G. I. Lau, W. T. Loo and L. W. C. Chow, “Neoadjuvant Chemotherapy for Breast Cancer Determined by Chemosensitivity Assay Achieves Better Tumor Response,” Biomedicine and Pharmacotherapy, Vol. 61, No. 9, 2007, pp. 562-565. doi:10.1016/j.biopha.2007.08.013
- D. S. Waldenmaier, A. Babarina and F. C. Kischkel, “Rapid in Vitro Chemosensitivity Analysis of Human Colon Tumor Cell Lines,” Toxicology and Applied Pharmacology, Vol. 192, No. 3, 2003, pp. 237-245. doi:10.1016/S0041-008X(03)00257-6
- R. D. Blumenthal, “An Overview of Chemosensitivity Testing,” Methods in Molecular Medicine, Vol. 110, 2005, pp. 3-18.
- B. Tegze, Z. Szállási, I. Haltrich, Z. Pénzváltó and Z. Tóth, “Parallel Evolution under Chemotherapy Pressure in 29 Breast Cancer Cell Lines Results in Dissimilar Mechanisms of Resistance,” PLoS One, Vol. 7, No. 2, 2012, Article ID: e30804. doi:10.1371/journal.pone.0030804
- A. Bagnato and L. Rosanó, “Understanding and Overcoming Chemoresistance in Ovarian Cancer: Emerging Role of the Endothelia Axis,” Current Oncology, Vol. 19, No. 1, 2012, pp. 36-38. doi:10.3747/co.19.895
NOTES
*Corresponding author.

