Pharmacology & Pharmacy
Vol.4 No.1(2013), Article ID:27514,4 pages DOI:10.4236/pp.2013.41014
Antidepressant Activities of the Methanol Extract, Petroleum Ether and Ethyl Acetate Fractions of Morus mesozygia Stem Bark
![]()
Department of Pharmacognosy, Faculty of Pharmacy, Olabisi Onabanjo University, Sagamu Campus, Nigeria.
Email: *adediwurajaiyesimi@gmail.com
Received November 24th, 2012; revised December 30th, 2012; accepted January 13th, 2013
Keywords: Morus mesozygia; Antidepressant Activity; Forced Swimming Test; Tail Suspension Test
ABSTRACT
Morus mesozygia Stapf (Moraceae) is the only Morus species indigenous to Tropical Africa. It is believed in traditional medicine that the plant exhibits several medicinal properties. The antidepressant-like activities of the crude methanol extract, petroleum ether and ethyl acetate fractions were investigated using the Forced Swimming Test (FST) and Tail Suspension Test (TST) models. The petroleum ether fraction at a dose of 140 mg/kg was the most effective in both the FST and TST when compared to the activity of the reference drug Imipramine (250 mg/kg) by reducing the immobility duration of the animals by 66.8% and 49.4% respectively.
1. Introduction
Morus mesozygia Stapf (Moraceae) is commonly referred to as the Black mulberry, [1] Ewe aye (Yoruba, Southwest Nigeria) [2] and it is the only species of the genus Morus native to Tropical Africa while Morus alba and Morus nigra were introduced into the region for their edible fruits and as food for silkworm [1]. M. mesozygia is a large tree of about 40 m high and characterised by white latex, ridges at the base and branchlets, whitish or glaborous hair.
Morus mesozygia is useful in construction, furniture, dug-out canoes and walking sticks. The wood is also useful as fuelwood and for charcoal making. In traditional folklore medicine, M. mesozygia is used in the treatment of arthritis, debility, stomach trouble, rheumatism, malnutrition, veneral disease and as a pain killer [1]. Previous studies reported the isolation of moracin R, S, T, U from the methanol extract of the stem of M. mesozygia and their antioxidant activities [3]. In addition, 3betaacetoxyurs-12-en-11-one, moracin Q, moracin T, artocarpesin, cycloartocarpesin were isolated from the stembark of Morus mesozygia while moracin KM, LM, and SC were isolated from the twigs [4].
The aim of this study is to investigate the antidepressant-like effects of the methanol extract, petroleum ether and ethyl acetate fractions of the stem bark of Morus mesozygia.
2. Materials and Methods
2.1. Plant Collection and Authentication
Fresh stembark samples of Morus mesozygia were collected in the month of August 2011 at Olokemeji in Ekiti State, Nigeria and authenticated at the Forestry Research Institute of Nigeria Ibadan (FRIN) by Mr Kola Adebanjo.
2.2. Drying and Extraction
The stem bark was air dried in the sun and milled into a fine powder. The powdered sample was extracted by cold extraction method using 80% methanol. The extract was concentrated under reduced pressure to give 112.6 g yield, the dried methanol extract was reconstituted in water and successively partitioned with petroleum ether and ethyl acetate to yield the pet ether and ethyl acetate fractions.
2.3. Animals
White albino rats of both sexes weighing between 98 and 180 g were obtained from the Department of Veterinary Physiology and Pharmacology, University of Ibadan, Nigeria. The animals were maintained at 25˚C, 12-hour light and dark cycle. The animals had access to food and water. The experiment was conducted in accordance with the International regulations for handling and use of laboratory animals.
2.4. Phytochemical Screening
Phytochemical screening was carried out using standard procedures [5,6].
2.5. Antidepressant Study
The Forced Swimming Test (FST) and Tail Suspension Test (TST) were the two animal models adopted in this study. These methods are known to reflect a state of despair or lowered mood in humans [7].
1) Forced Swimming Test (FST)
The Forced Swimming Test adopted from the method of Porsolt was used in this study [8,9]. Five animals (n = 5) were used per group.
Group A: Rats received 1 ml of distilled water each.
Group B: Rats received 25 mg/kg of Imipramine.
Group C: Rats received 140 mg/kg of Ethyl acetate fraction.
Group D: Rats received 140 mg/kg of Petroleum ether fraction.
Group E: Rats received 140 mg/kg of methanolic crude extract.
Thirty minutes after administering extract, fractions, reference drug and control, individual rats were forced to swim in an open cylindrical container (Diameter 20 cm, height 30 cm) containing 20 cm of water at 25˚C ± 1˚C. The total duration of immobility was recorded in a sixminute period. The rats were judged to be immobile when ceased struggling and remained floating motionless in the water, making only those movements necessary to keep their heads above water. A decrease in the duration of immobility was indicative of an antidepressant-like effect [8].
2) Tail Suspension Test
The tail suspension method used in this study was similar to those described by Steru et al., 1985 [10]. The rats were suspended upside down on a metal rod at a height of 50 cm from the ground with the help of an adhesive tape placed approximately 1 cm from the tip of the tail. The animals initially tried to escape by making vigorous movements but became immobile after a while. The animals were subjected to this kind of unavoidable and inescapable stress to reflect behavioural despair which in turn may reflect depressive disorders in humans. The total duration of immobility was rated within a 6 minutes period with each rat used only once.
2.6. Statistical Analysis
The results are expressed as mean ± SEM and as a percentage (%) reduction in the immobility of the animals.
3. Results (Tables 1-2)
Table 1. Phytochemical screening of stembark of Morus mesozygia.

+ = Present; − = Absent.
Table 2. Antidepressant-like effects of Morus mesozygia stem bark extract and fractions.
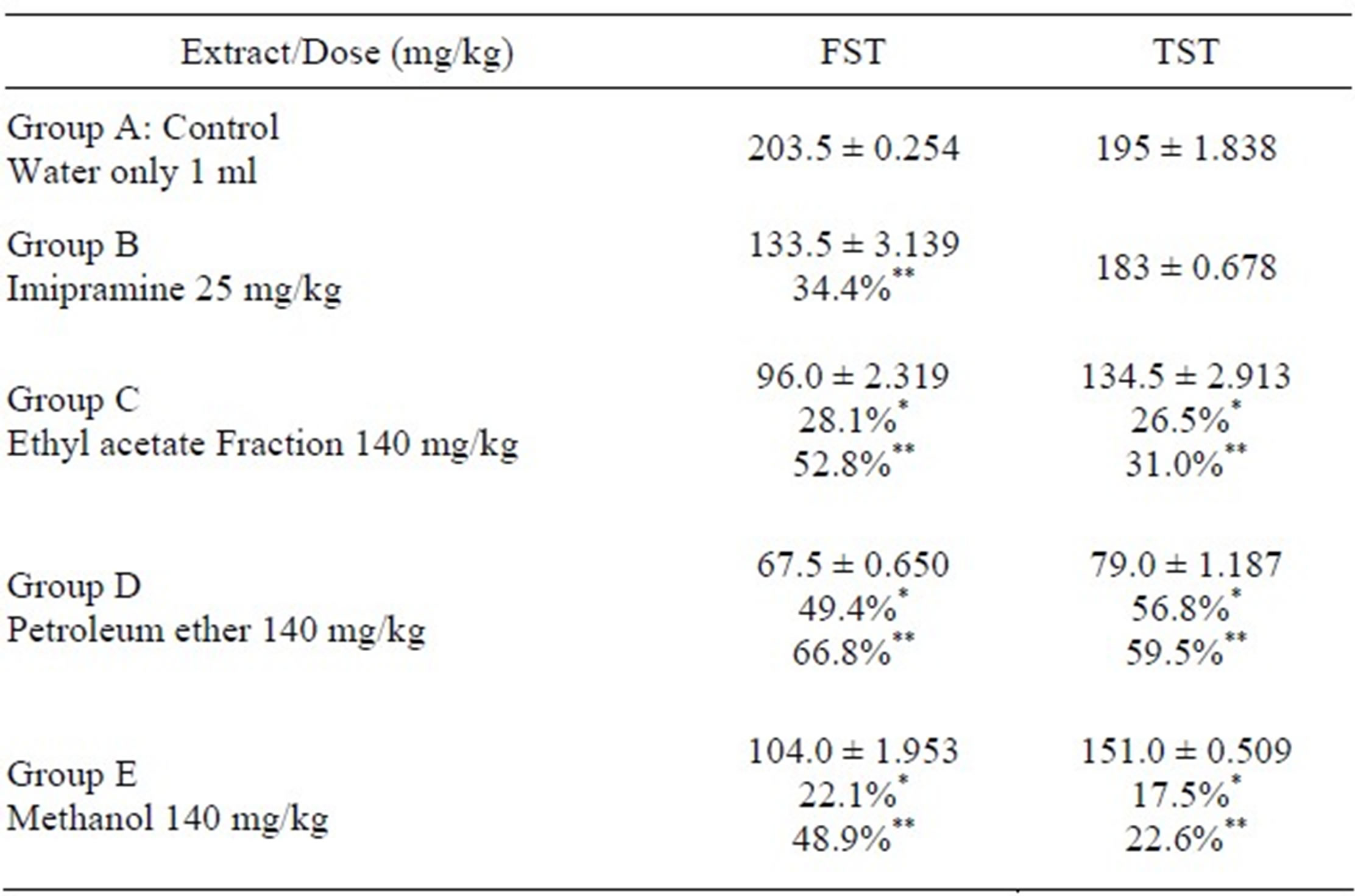
*Compared to reference drug Imipramine; **Compared to untreated control.
4. Discussion
Depression a chronic condition in clinical practice is associated with psychosocial and physical impairment [11]. This state of health has been attributed to deficiency in the functions of cerebral monoaminergic transmitters such as norepinephrine and/or dopamine located at synapses [12]. The World Health Organization has indicated that Depression will become the second condition leading to premature death or disability worldwide by 2020 [13].
In a previous study by Song et al., 2009, the phytochemical profile of several species of several Morus species showed that members of the genus Morus are rich in alkaloids, flavonoids and polyphenols [14].
In this study, the phytochemical screening of the stem bark of Morus mesozygia showed the presence of saponin, cardiac glycosides, tannins, alkaloids, flavonoids and anthraquinone.
The Tail Suspension Test and Forced Swimming Test are considered as animal models for antidepressant drug screening [15,16]. These models mimic the unipolar type of depression in human which is characterized by depressive mood, difficulty in sleeping, fatigue, difficulty in making decisions and feeling worthless. These models are considered to be sensitive and relatively specific to all major classes of antidepressant drugs like tricyclics, selective serotonin reuptake inhibitors and monoamine oxidase inhibitors [8,17].
In this study, the effect of the ethyl acetate and petroleum ether fractions at a dose of 140 mg/ml were more effective than the reference drug Imipramine (25 mg/ml) when compared to the untreated control. The ethyl acetate fraction exhibited 28.1% and 26.5% reduction in immobility in the FST and TST when compared to Imipramine, 52.8% and 31.0% when compared to the untreated control. The petroleum ether fraction reduced immobility by 49.4% and 56.8% antidepressant effect when compared to Imipramine, 66.8% and 59.5% when compared to the untreated control in the FST and TST respectively.
In the FST and TST, the petroleum ether fraction was the most effective. The antidepressant-like effects exhibited by these fractions (petroleum ether and ethyl acetate) in the animal models used were not significantly different from each other. They however, exhibited a more pronounced effect than Imipramine (25 mg/kg) the reference drug.
Previous studies have reported the antidepressant-like effects of flavonoids such as quercetin glycosides [18- 20].
The antidepressant-like effect exhibited by the stem bark of Morus mesozygia might be due to the presence of the alkaloids, saponins and flavonoids. Flavonoid glycosides have been reported to exhibit antidepressant-like effects by being hydrolysed to their aglycones by mucosal and bacterial enzymes in the intestines which are then converted to conjugated metabolites during the absorption process [21,22] which then protects the brain function from central nervous system (CNS) disturbance [23].
Though the exact group of compound (s) responsible for the antidepressant-like effect is unknown, the pronounced antidepressant effect might be exerted by protecting brain function from CNS disturbance, [23] due to adaptogenic properties, by reducing NGF levels in the hippocampus which can contribute to the antidepressant-like effect on FST [24] or by exhibiting corticosteroid—like effects on the modulation of nerve transmission by altering the availability of neurotransmitters [25].
REFERENCES
- H. M. Burkill, “The Useful Plants of West Tropical Africa,” Economic Botany & Ethnobotany, Vol. 1, 1985, p. 319.
- Z. O. Gbile, “Vernacular Names of Nigerian Plants (Yoruba),” Forestry Research Institute of Nigeria, Ibadan, 1984.
- G. D. W. F. Kapche, C. D. Fozing, J. H. Donfack, G. W. Fotso, D. Amadou, A. N. Tchana, M. Bezabih, P. F. Moundipa, B. T. Ngadjui and B. M. Abegaz, “Prenylated Arylbenzofuran Derivatives from Morus mesozygia with Antioxidant Activity,” Phytochemistry, Vol. 70, No. 2, 2009, pp. 216-221.
- G. W. D. Kapche, D. Amadou, P. Waffo-Teguo, J. H. Donfack, C. D. Fozing, D. Harakat, A. N. Tchana, J. M. Merillon, P. F. Moundipa, B. T. Ngadjul and B. M. Abegaz, “Hepatoprotective and Antioxidant Arylbenzofurans and Flavonoids from the Twigs of Morus mesozygia,” Planta Medica, Vol. 77, No. 10, 2011, pp. 1044- 1047
- W. C. Evans, “Pharmacognosy,” 15th Edition, Saunders, London, 2009, p. 585.
- J. B. Harborne, “Method of Extraction and Isolation,” Phytochemical Methods, 3rd Edition, Chapman and Hall, London, 1998, pp. 60-66.
- V. K. Sharma, N. S. Chauhan, S. Lodhi and A. K. Singhai, “Anti-Depressant Activity of Zizyphus xylopyrus,” International Journal of Phytomedicine, Vol. 1, 2009, pp. 12- 17. doi:10.5138/ijpm.2009.0975.0185.05788
- R. D. Porsolt, M. Le Pichon and M. Jalfre, “Depression: A New Animal Model Sensitive to Antidepressant Treatments,” Nature, Vol. 266, No. 5604, 1977, pp. 730-732.
- R. Porsolt, G. Anton and M. Jafre, “Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments,” European Journal of Pharmacology, Vol. 47, No. 4, 1978, pp. 379-391.
- L. Steru, R. Chermat, B. Thierry and P. Simon, “The Tail Suspension Test: A New Method for Screening Antidepressants in Mice,” Psychopharmacology, Vol. 85, No. 3, 1985, pp. 367-370.
- M. A. Whooley and G. E. Simon, “Managing Depression in Medical Outpatients,” New England Journal of Medicine, Vol. 343, 2000, pp. 1942-1950. doi:10.1056/NEJM200012283432607
- J. J. Schildkraut, “The Catecholamine Hypothesis of Effective Disorders: A Review of Supporting Evidence,” American Psychiatric Association, Vol. 122, No. 5, 1965, pp. 509-522.
- World Health Organization, “WHO Director-General Unveils New Global Strategies for Mental Health,” Press Release WHO/99-67, 1999. http://www.who.int/inf-pr-1999/en/pr99-67
- W. Song, H. Wang, P. Bucheli, P. Zhang, D. Wel and Y. Lu, “Phytochemical Profiles of Different Mulberry (Morus sp) Species from China,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 19, 2009, pp. 9133- 9140. doi:10.1021/jf9022228
- S. K. Bhattacharya, K. S. Satyan and M. Ramanathan, “Experimental Methods for Evaluation of Psychotropic Agents in Rodents: II Antidepressants,” Indian Journal of Experimental Biology, Vol. 37, No. 2, 1999, pp. 117-123.
- R. Daudt, G. L. Von Poser, G. Neves and S. M. K. Rates, “Screening for Antidepressant Activity of Some Hypericum from South Brazil,” Phytotherapy Research, Vol. 14, No. 5, 2000, pp. 344-346.
- M. J. Detke, M. Rickels and I. Lucki, “Active Behavior in the Rat Forced Swimming Test Differently Produced by Serotonrgic and Noradrenergic Antidepressants,” Psychopharmacology, Vol. 121, No. 1, 1995, pp. 66-72.
- V. Butterweck, G. Jurgenliemk, A. Nahrstedt and H. Winterhoff, “Flavonoids from Hypericum Perforatum Show Antidepressant Activity in the Forced Swimming Test,” Planta Medica, Vol. 66, No. 1, 2000, pp. 3-6.
- V. Butterweck, S. Nishibe, T. Saasaki and M. Uchida, “Antidepressant Effects of Apocynum venetum Leaves in Forced Swimming Test,” Biological & Pharmaceutical Bulletin, Vol. 24, No. 7, 2001, pp. 848-851.
- M. Nolder and K. Schotz, “Rutin in Essential for the Antidepressant Activity of Hypericum perforatum Extracts in Forced Swimming Test,” Planta Medica, Vol. 68, No. 7, 2002, pp. 577-580.
- V. D. Bokkenheuser, C. H. Shackleton and J. Winter, “Hydrolysis of Dietary Flavonoids Glycosides by Strains of Intestinal Bacterioides from Humans,” Biochemical Journal, Vol. 248, 1987, pp. 953-956.
- T. Walle, “Absorption and Metabolism of Flavonoids,” Free Radical Biology & Medicine, Vol. 36, No. 7, 2004, pp. 829-837.
- P. M. Umadevi, S. S. Jennifer and S. Subakanmani, “Evaluation of Antidepressant Like Activity of Curcubita Pepo Seed Extracts in Rats,” International Journal of Current Pharmaceutical Research, Vol. 3, No. 1, 2011, pp. 108-113.
- K. Yamaura, N. Nakayama, M. Shimada, Y. Bi, H. Fukata and K. Ueno, “Antidepressant-Like Effects of Young Green Barley Leaf (Hordeum vulgare L.) in the Mouse Forced Swimming Test,” Pharmacognosy Research, Vol. 4, No. 1, 2012, pp. 22-26.
- D. Tsang, H. W. Yeung, W. W. Tso and H. Peck, “Ginseng Saponins: Influence on Neurotransmitter Uptake in Rat Brain Synaptosomes,” Planta Medica, Vol. 51, No. 3, 1985, pp. 221-224.
NOTES
*Corresponding author.

