Advances in Materials Physics and Chemistry
Vol.3 No.1(2013), Article ID:29310,5 pages DOI:10.4236/ampc.2013.31013
Synthesis and Characterization of Ferrate(VI) Alkali Metal by Electrochemical Method
1Laboratoire de Chimie de La matière Condensée (LCMC), Faculté des Sciences et Techniques, Université Sidi Mohammed Ben Abdellah, Fes, Morocco
2Laboratoire de Physique et Mécanique des Matériaux, Faculté des Sciences et Techniques Beni Mellal, Université Sultan Moulay Slimane, Beni Mellal, Morocco
Email: elmaghraabdellatif@yahoo.fr
Received December 27, 2012; revised February 11, 2013; accepted February 24, 2013
Keywords: Ferrate; Bactericidal; Oxidant; Flocculants; Coagulant; Conductivity; Cathode
ABSTRACT
This works aims at preparing at room stable Na2FeO4 and tracking its degradation over time. The synthetic, during this step, was carried out by electrochemical method. The latter was given maximum focus because of its simplicity and the high degree of purity of the resulting product with respect to wet and dry method. This paper reviews the development of the electrochemical method applied to the synthesis of stable at room Na2FeO4, optimizing the parameters impacting the performance of the oxidation of iron(II) in to iron(VI) in alkaline NaOH, saturated at a temperature of 61˚C and a current density of 1.4 A/dm2, in order to simplify the synthesis process, to minimize the cost and to improve the production of iron(VI) to meet the growing demand of ferrate(VI) useful for water treatment. The supervision of the degradation of synthesized Na2FeO4 shows its stability over a period of 10 months, which makes storage and transport easier. The phases obtained were characterized by IR spectrometry, X-ray, Mössbauer, spectroscopy and thermogravimeric analysis.
1. Introduction
Iron compounds in the oxidation state (VI) have the advantage of being powerful antioxidants and bactericides, which explains their particular interest in water treatment. Moreover, their results in reduction of ferric hydroxide, their flocculating power contributes to the elimination of organic pollutants, minerals (hydrocarbons, heavy metals, radioactive isotopes...) and industrial effluents.
The synthesis of ferrate(VI) appears to be very delicate, because of the instability that gives them their high oxidizing power. Although the existence of alkaline ferrate has been testified for a century [1,2]. Several efforts have been made to synthesize the solid sodium ferrate [3-7]. Difficulties were encountered in isolating the solid product from each of the resulting solutions and stabilizing it.
The Na2FeO4 phase was synthesized by electrochemical way. Its oxidizing power makes it possible to use it as an oxidizing agent and a disinfectant in water treatment. It first reacts as iron(VI) causing an oxidation process during which the iron(VI) is reduced to Fe(III) that is itself used in the treatment of waste water to precipitate phosphate. The induced oxidation is not accompanied by unwanted byproducts. The aim of our work is to synthesize compounds based on iron(VI)  stable at room temperature and replace potassium hydroxide by sodium hydroxide to obtain a significant reduction in the cost of synthesis of these ferrates. The electrochemical method used can provide a useful way for the industrial mass production of iron(VI).
stable at room temperature and replace potassium hydroxide by sodium hydroxide to obtain a significant reduction in the cost of synthesis of these ferrates. The electrochemical method used can provide a useful way for the industrial mass production of iron(VI).
2. Experimental
2.1. Synthesis
The first electrochemical synthesis of ferrate(VI) is due to Poggendorff (Poggendorff, 1841) [8]. This method is the easiest way to obtain the sodium ferrate in solution without impurities. The principle of the synthesis involves the oxidation of pig iron or iron salts in alkaline solutions using concentrated NaCl as a stabilizer. Figure 1 shows the experimental synthesis.
Next the various tests made by G. Grube [9,10], in concentrated NaOH environment by varying the Current density and temperature and the electrolysis time. During these tests, we found out that the performance of the iron oxidation varies depending on the current density, the temperature and the electrolysis time, according to Figure 2.

Figure 1. Schematic representation of the electrochemical cell for the synthesis of ferrate.

Figure 2. Performance of the oxidation of iron as a function of the current density I at T = 61˚C in concentrated NaOH for I = 1.4 A/dm2. Performance reaches a maximum value of 60%.
The increase in temperature leads to higher yields but with a maximum at T = 61˚C Figure 3. Indeed, it catalyzes the oxidation of iron. Regarding the electrolysis time, it is obvious, from a first abort, that the number of moles of ferrates produced will increase if the reaction time is longer. But, alongside with this, we must not forget that we operate in aqueous media and that in contact with water, ferrate is reduced to form a precipitate of ferric hydroxyl according to the reaction:

In fact, from Figure 4, we can notice that beyond about an hour, the rate of decomposition 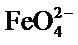 exceeds that of synthesis, since the over all performance drops.
exceeds that of synthesis, since the over all performance drops.
The reactions involved are:
Anode Simultaneous oxidation of iron and solvent according
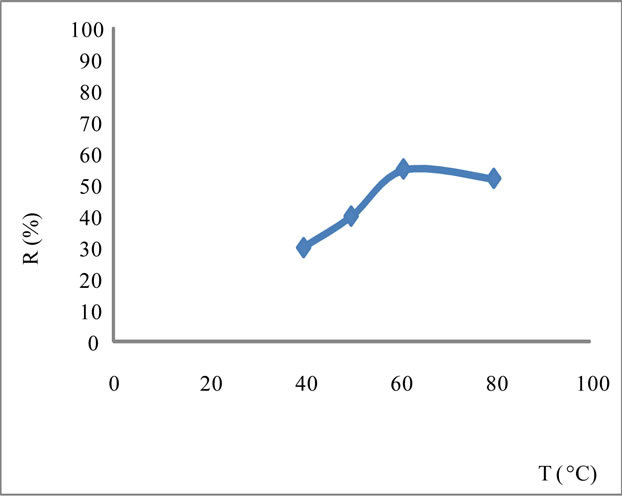
Figure 3. Yield of the oxidation of iron depending on temperature T in NaOH saturated with I = 1.4 A/dm2.

Figure 4. Effect of electrolysis time on the efficiency of the anodic oxidation of iron at T = 61˚C in concentrated NaOH with I = 1.4 A/dm2.
to the reaction:
 è
è
 è
è
To obtain sufficiently high concentration ferrate, we use another source of Fe(III), that is the ferric salt FeCl3, 6H2O according to the following reaction:
 é
é
 Formation process Trivalent iron reacts with the OH− to form an oxohydroxyl complex de FexOy·nH2O type which will then be oxidized electrochemically in the presence of ferrate halide (NaCl).
Formation process Trivalent iron reacts with the OH− to form an oxohydroxyl complex de FexOy·nH2O type which will then be oxidized electrochemically in the presence of ferrate halide (NaCl).
Cathode Only the reduction in hydrogen of the solvent was held.
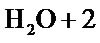 é
é
2.2. Characterizations
2.2.1. X-Ray Diffraction
Measures by a radiation ray diffractometer CuK of a compound of ferrate powder Na2FeO4 Figure 5 proves the crystal structure of ferrate [11,12] and demonstrates the existence of an isomorphism with K2FeO4 and BaFeO4 as found by Stuart Licht, Vera. Naschitz and collaborators [13]. From the analytical point of view, the X-ray diffraction is one of the means used to verify the presence of ferrate.
The spectrum obtained on Na2FeO4 bears a strong similarity with that of isomorphous compounds. There is a splitting of the lines corresponding to planes (102), (202), (013), (200), (002), (004) [14-16].
2.2.2. Infrared Spectrum
The appearance of an infrared spectrum is related to the symmetry of the molecule or study group. We expected for , with tetrahedral structure, to find: first, the fundamental bands characteristic of a symmetry 2d: either bands
, with tetrahedral structure, to find: first, the fundamental bands characteristic of a symmetry 2d: either bands  and
and  from the two degenerate modes of vibration: the symmetric stretching and angular deformation within the tetrahedron resulting in inactive modes in infrared absorption, bands
from the two degenerate modes of vibration: the symmetric stretching and angular deformation within the tetrahedron resulting in inactive modes in infrared absorption, bands  and
and  must be absent from the spectra [17]. On the other hand, a similarity is among infrared spectra of isomorphism series [18]. The presence of a band
must be absent from the spectra [17]. On the other hand, a similarity is among infrared spectra of isomorphism series [18]. The presence of a band  and a triplet for
and a triplet for 

Figure 5. X-ray diffractometer of Na2FeO4.
(elongation of the tetrahedron) have led W. Griffith [19] to consider a lower symmetry in 2d, very close to 2s the  anion.
anion.
IR spectroscopy is a quantitative method for the determination of iron(VI) compounds in the ferrate. The shape of the spectra is related to the symmetry of the molecule or  groups (tetrahedral structure) the IR spectrum of Na2FeO4. Figure 6 showed a similar pace in the field of high frequency to similar that obtained (mode 825 cm−1 and 780 cm−1) by P. Tarte and G. Nizete [20].
groups (tetrahedral structure) the IR spectrum of Na2FeO4. Figure 6 showed a similar pace in the field of high frequency to similar that obtained (mode 825 cm−1 and 780 cm−1) by P. Tarte and G. Nizete [20].
The IR results support those obtained by XRD.
2.2.3. ATG Spectrum
In general, two stages of decomposition were obtained in the TGA curve up to 500˚C Figure 7. A first one above 100˚C corresponding to the evolution of water weakly adsorbed by the sample and a second step between 210˚C and 310˚C corresponding to the release of O2 Scholder et al. [21] and [22].
The two stages of decomposition were accompanied by endothermic heat effects as measured by TGA.
3. Tracking the Degradation of Ferrates as Function of Time
Analysis by Mössbauer Spectroscopy
The Mössbauer effect highlights the absorption of apho-
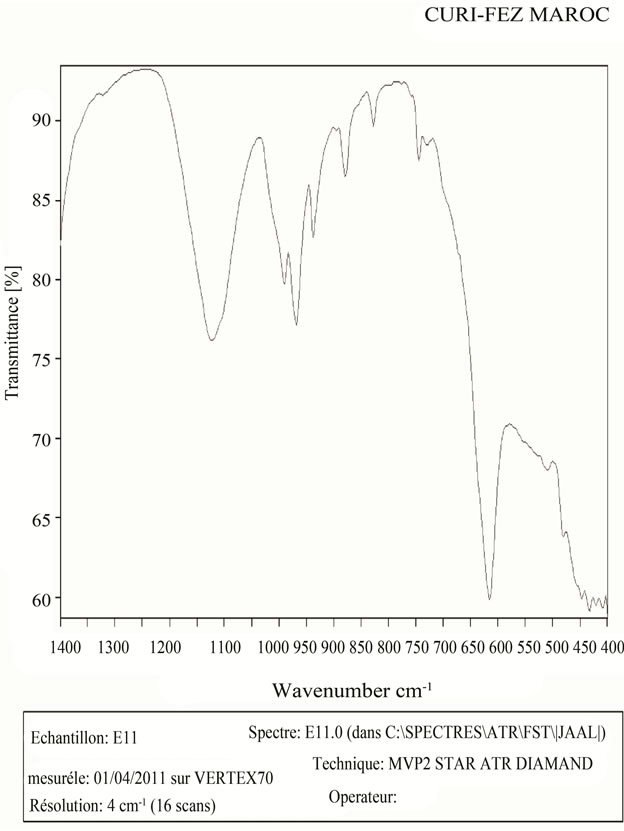
Figure 6. Na2FeO4 infra-red spectrometer.

Figure 7. TGA spectrum of Na2FeO4.

Figure 8. Mössbauer Na2FeO4 spectrometer after ten months of storage.
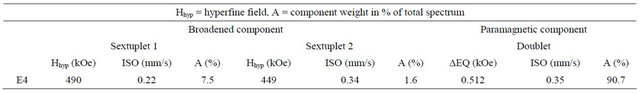
Table 1. Hyperfine parameters deduced from the calculation of the spectrum after ten months of storage.
ton Y by a nucleus of 57Fe (present at a rate of about 2.6 percent in the natural iron): we varied gradually the energy of the photon emitted by variation of the speed of the source (57Co. When it reaches a value equal to the difference between the energy level of the core in its ground state (l = 1/2) and its level in the excited state (l = 3/2) the photon is absorbed. This phenomenon results in a peak on the spectrum.
Mössbauer spectroscopy also helped to highlight the existence of a magnetic order at low temperature [23-27]. Na2FeO4 characterization by Mössbauer spectroscopy, after ten months of storage at room temperature, reveals a degradation of iron(VI) over time according to the Mössbauer spectrum Figure 8. We have a broad magnetic component calculated by the superposition of two sextuplets and a paramagnetic component adjusted by a paramagnetic doublet. The hyperfine parameters deduced from the calculation of the spectrum are given in Table 1.
This allows us to visualize the oxidation of iron and therefore control the rate of iron(VI) over time. This degradation is manifested on the spectrum by the isomer shift of the peaks to 0.22 mm/s for sextuplets 1 and 0.34 for sextuplets 2 (see Table 1) while the sextuplet iron(VI) comes close to −1 [23-27].
This isomer shift is due to degradation of Fe(VI) to iron(III) because of moisture.
4. Conclusions
From the results, it’s possible to synthesize at room stable sodium ferrate Na2FeO4 electrochemically at a temperature of 61˚C and a current density of I = 1.4 A/dm2 in an alkaline medium saturated NaOH.
Infrared spectroscopy shows that we are dealing with a compound containing the  group.
group.
Mössbauer spectroscopy of iron allowed us to visualize the oxidation of iron and therefore to control the rate of iron(VI), and track its degradation in iron(III) over time.
The XR spectrum of Na2FeO4 is isomorphic to that given by K2FeO4 literature.
The spectrum shows a peak ATG at 100˚C corresponding to the release of water and a peak at 295˚C corresponding to the decomposition of Na2FeO4.
REFERENCES
- B. Von Helferich, K. lang, Zeit. Anorg. Chem. Vol. 263, 1950, p. 169.
- R. J. Audette and J. W. Quail, “Potassium, Rubidium, Cesium, and Barium Ferrates(VI). Preparations, Infrared Spectra, and Magnetic Susceptibilities,” Inorganic Chemistry, Vol. 11, No. 8, 1972, p. 1904. doi:10.1021/ic50114a034
- E. F. Fremy, “Recherches sur les Acides Mètalliques,” Annales de Chimie et de Physique, 1844, pp. 361-382.
- W. Foster, “The Action of Alkaline Hypobromite on Oxamide, Urea and Potassuim Ferrocyanide,” Journal of Chemical Society, Vol. 35, 1879, pp. 119-124. doi:10.1039/ct8793500119
- Blattner, “Action des Oxydes Mètalliques sur les Hypochlorites a Lcalino-Terreux,” Bulletin de la Société Chimique, Vol. 7, 1892, pp. 700-708.
- M. Muspratt and S. Smith, “Some Experiments upon High Strength Hypochlorite Solutions,” Journal of the Society of Chemical Industry, Vol. 17, 1898, pp. 1096-1100.
- W. F. Wagner, J. R. Gump and E. N. Hurt, “Factors Affecting Stability of Aqueous Potassium Ferrate(VI) Solutions,” Analytical Chemistry, Vol. 24, No. 9, 1952, pp. 1497-1498. doi:10.1021/ac60069a037
- J. C. Poggendorf, Pogg. Ann, Vol. 54, 1841, p. 161.
- G. Grube and H. Gmelin, “Der Einfluss Überlagerten Wechselstromes auf die Anodische Ferratbildung,” Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie, Vol. 26, 1920, p. 153.
- G. Grube, “Die Passivitat der Metalle bei Anodischer Polarisation,” Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie, Vol. 33, No. 9, 1927, p. 389.
- B. Helferich and K. Lang, “Über Salze der Eisensäure Mit 3 Abbildungen,” Zeitschrift für Anorganische und Allgemeine Chemie, Vol. 263, No. 4, 1950, pp. 169-174. doi:10.1002/zaac.19502630404
- H. Von Krebs, “Über die Struktur des Kaliumferrats und des Bariumferrats Mit 2 Abbildungen,” Zeitschrift für Anorganische und Allgemeine Chemie, Vol. 263, No. 4, 1950, pp. 175-176. doi:10.1002/zaac.19502630405
- S. Licht, V. Naschitz, L. Halperin , N. Halperin, L. Lin, J. Chen, S. Ghosh and B. Liu, “Analysis of Ferrate(VI) Compounds and Super-Iron Fe(VI) Battery Cathodes: FTIR, ICP, Titrimetric, XRD, UV/VIS, and Electrochemical Characterization,” Journal of Power Sources, Vol. 101, No. 2, 2001, pp. 167-176. doi:10.1016/S0378-7753(01)00786-8
- Y. L. Wang, S. H. Ye, Y. Y. Wang, J. S. Cao and F. Wu, “Structural and Electrochemical Properties of a K2FeO4 Cathode for Rechargeable Li Ion Batteries,” Electrochimica Acta, Vol. 54, No. 16, 2009, pp. 4131-4135. doi:10.1016/j.electacta.2009.02.053
- W. C. He, J. M. Wang, H. B. Shao, J. Q. Zhang and C.-N. Cao, “Novel KOH Electrolyte for One-Step Electrochemical Synthesis of High Purity Solid K2FeO4: Comparison with NaOH,” Electrochemistry Communications, Vol. 7, No. 6, 2005, pp. 607-611. doi:10.1016/j.elecom.2005.04.011
- R. J. Audette and J. W. Quail, “Potassium, Rubidium, Cesium, and Barium Ferrates(VI). Preparations, Infrared Spectra, and Magnetic Susceptibilities,” Inorganic Chemistry, Vol. 11, No. 8, 1972, pp. 1904-1908.
- N. Becarud, C. Duval. C. R. Acad. Sci, Vol. 257, 1963, p. 1930.
- F. Gonzalesvilchez and W. Griffith, “Transition-Metal TetraOxo-Complexes and Their Vibrational Spectra,” Journal of Chemistry Society, Dalton Transactions, No. 13, 1972, pp. 1416-1421. doi:10.1039/dt9720001416
- W. Griffith, “Infrared Spectra of Tetrahedral Oxyanions of the Transition Metals,” Journal of the Chemical Society A: Inorganic, Physical, Theoretical, 1966, pp. 1467- 1468. doi:10.1039/j19660001467
- P. Tarte and G. Nizet, “Etude Infra-Rouge de Quelques Composés du Type K2SO4 et BaSO4,” Spectrochimica Acta, Vol. 20, No. 3, 1964, pp. 503-513. doi:10.1016/0371-1951(64)80045-X
- R. Scholder, H. Bunsen, F. Kin, W. Zeiss and Z. Anorg, “Zur Kenntnis der Ferrate(VI),” Zeitschrift für Anorganische und Allgemeine Chemie, Vol. 282, No. 1-6, 1955, pp. 268-279. doi:10.1002/zaac.19552820129
- R. Scholder, “Recent Investigation on Oxometallates and Double Oxides,” Angewandte Chemie, Vol. 1, No. 4, 1962, pp. 220-224. doi:10.1002/anie.196202202
- A. Ito and K. Ono, “Mössbauer Study of Fe6+ in Potassium Ferrate, K2FeO4,” Journal of the Physical Society of Japan, Vol. 26, 1969, p. 1548. doi:10.1143/JPSJ.26.1548
- T.Shinjo, T. Ichida and T. Takada, “Internal Magnetic Field at Fe in57 Hexavalent States,” Journal of the Physical Society of Japan, Vol. 26, 1969, p. 1547. doi:10.1143/JPSJ.26.1547
- T. Shinjo, T. Ichida and T. Takada, “Fe57 Mössbauer Effect and Magnetic Susceptibility of Hexavalent Iron Compounds; K2FeO4, SrFeO4 and BaFeO4,” Journal of Physical Society of Japan, Vol. 29, No. 1, 1970, pp. 111- 116. doi:10.1143/JPSJ.29.111
- G. Hoy and M. Corson, “Critical Slowing Down of Spin Fluctuations in K2FeO4,” Journal of Magnetism and Magnetic Materials, Vol. 15, No. 18, 1980, pp. 627-628. doi:10.1016/0304-8853(80)90693-9
- F. Menil, “Systematic Trends of the 57Fe Mössbauer Isomer Shifts in (FeOn) and (FeFn) Polyhedra. Evidence of a New Correlation between the Isomer Shift and the Inductive Effect of the Competing Bond T-X (→Fe) (Where X Is O or F and T Any Element with a Formal Positive Charge),” Journal of Physics and Chemistry of Solids, Vol. 46, No. 7, 1985, pp. 763-789. doi:10.1016/0022-3697(85)90001-0

