Agricultural Sciences
Vol.4 No.6A(2013), Article ID:33753,8 pages DOI:10.4236/as.2013.46A011
Dietary selenium requirement of yellowtail kingfish (Seriola lalandi)
![]()
Department of Environment and Agriculture, Curtin University, Perth, Australia; *Corresponding Author: trungkyle@yahoo.com
Copyright © 2013 Ky Trung Le, Ravi Fotedar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 19 April 2013; revised 20 May 2013; accepted 12 June 2013
Keywords: Selenium Requirement; Vibrio anguillarum; Yellowtail Kingfish
ABSTRACT
The dietary selenium (Se) requirement of yellowtail kingfish (Seriola lalandi) in normal and infected conditions was investigated. The fish were fed one of five experimental diets; a control un-supplemented diet (3.35 mg/kg Se) or diets supplemented with Se to provide 4.86, 5.38, 5.85 or 6.38 mg/kg Se. After feeding for 6 weeks, the fish were challenged by Vibrio anguillarum immersion and then observed for 4 weeks. Supplementation of Se had no effect on feed intake, feed conversion ratio and survival over 6 weeks of feeding; however, it significantly increased growth and Se content in muscle tissues. The optimal Se level for maximal growth of yellowtail kingfish estimated by second order regression was 5.56 mg/kg. Following the bacterial challenge, the immune-stimulating effects of Se were demonstrated in lysozyme and bactericidal activities, and there was a corresponding increase in survival and antibody response by supplementation of Se at ≥2 mg/kg (measured Se of ≥5.38 mg/kg). Under normal and infectious conditions, antioxidant capacity of fish measured as glutathione peroxidase activity increased by supplementation of Se. During post-challenge period, haematocrits were higher in the fish fed Se supplemented diets than the fish fed the control diet, while more macrophage aggregates were seen in the control group than in the others. Furthermore, there was evidence of myopathy in fish fed the diet without Se supplementation. Therefore, the results indicated that the optimal dietary Se requirement of yellowtail kingfish is 5.56 mg/kg.
1. INTRODUCTION
The need for knowledge of nutritional requirements of yellowtail kingfish (Seriola lalandi) has increased recently as its aquaculture activity is expanding and intensifying. In the last few years, the research in the area of yellowtail kingfish nutrition has been conducted to refine practical diet formulations. However, the research has focused mainly on requirements for protein, energy and various sources of lipid [1,2]. Up to date there is no published information on the mineral requirements of yellowtail kingfish.
One mineral that has been known as an essential trace element for normal growth and physiological function of animals, including fish is selenium (Se) [3]. Se is a component of the enzyme glutathione peroxidase, which plays an important role in protecting cell membranes against oxidative damage [4]. It is also required for the efficient functioning of many components of the immune system [5,6]. This is especially important in intensive fish farming as fish often suffer from multiple microbial infections.
Recently, the outbreaks of diseases in intensive aquaculture operations have increased and are being recognized as a significant limitation on sustainable aquaculture. Being the most common in many aquaculture systems, vibriosis of Vibrio anguillarum has been found in over 42 species of fish [7] and is described as a serious pathogen affecting cultured marine fish worldwide [8]. Therefore, protecting cultured fish from this disease is essential for the expansion and sustainability of the aquaculture industry.
The nutritional requirements of fish have been primarily based on growth and deficiency symptoms [9] rather than on health status indicators such as immune responses and disease resistance. The objective of this study was to estimate the optimal Se requirement of juvenile yellowtail kingfish for maximal growth as well as after challenging with V. anguillarum.
2. METERIALS AND METHODS
All experimental work was approved by the Curtin University Animal Ethics Committee and performed according to the Australian Code of Practice for the care and use of animals for scientific purposes. Chemicals used were analytical grade obtained from Thermo Fisher Scientific, Scoresby, VIC, Australia, unless otherwise stated.
2.1. Experimental Diets
Five experimental dietary treatments were designed. One treatment comprised of the un-supplemented basal diet (control) and the others were supplemented with Se at 1.5, 2, 2.5 and 3 mg/kg. A basal mash of a commercially available yellowtail kingfish diet (Marine CST, Ridley AgriProducts, Melbourne, VIC, Australia) without any supplementation of Se was used to prepare the experimental diets. This mash, containing (mean ± SD, n = 3) 46.42% ± 1.20% protein, 15.05% ± 0.16% lipid, 91.48% ± 0.05% dry matter, 9.56% ± 0.16% ash and provided 21.68 ± 0.34 MJ/kg energy, was extruded into 3 mm pellets. Following extrusion, the necessary quantity of Se from Se-yeast (Selplex®, Alltech, Nicholasville, KY, USA) was top coated to the experimental pellets with gelatin (Davis Gelatine, Christchurch, New Zealand) to form the five experimental diets. The measured Se concentrations in each diet were (mg/kg; mean ± SD, n = 3); 3.35 ± 0.01, 4.86 ± 0.02, 5.38 ± 0.03, 5.85 ± 0.03 and 6.38 ± 0.02 for the un-supplemented basal diet, diets supplemented with Se at 1.5, 2, 2.5 and 3 mg/kg, respectively. The selected Se levels were based on our previous studies, in which the diet supplemented at 2 mg/kg Se produced the beneficial outcomes for yellowtail kingfish in comparison to un-supplemented diet, supplemented at 1 or 4 mg/kg.
2.2. Growth Trial
Yellowtail kingfish were supplied by the Australian Centre for Applied Aquaculture Research, Fremantle, WA, Australia and brought to the Curtin Aquatic Research Laboratory (CARL), Curtin University. The fish were group weighed and stocked into each of 15 experimental 300-L tanks at a density of 12 fish/tank. Total weight of fish in each tank was 223.98 ± 1.23 g (mean ± SD), with an average individual weight of 18.66 ± 0.10 g (mean ± SD). The tanks were filled up with seawater (salinity 35 ppt) and supplied with constant aeration and pure oxygen (oxygen compressed, BOC, Perth, WA, Australia). Each tank had an external bio-filter (Fluval 406, Hagen, Italy) running continuously to create a recirculating system at a rate of approximately 900 L/h. Half of the water was changed twice weekly. Water temperature, pH and dissolved oxygen were measured daily using digital pH/mV/˚C and dissolved oxygen meters (CyberScan pH 300 and CyberScan DO 300, Eutech Instruments, Singapore). During the trial, water temperature, pH and dissolved oxygen were maintained at (mean ± SD) 21.82˚C ± 0.80˚C, 7.56 ± 0.24 and 6.56 ± 0.38 mg/L, respectively.
Each dietary treatment was randomly assigned to three replicate tanks. Fish were fed to apparent satiation twice a day at 0800 and 1600 h for 6 weeks.
2.3. Bacterial Preparation and Challenge
Vibrio anguillarum was obtained from Bacteriology Laboratory, Department of Agriculture & Food, Perth, WA, Australia. The bacteria were cultured in tryptone soya broth (Oxoid, Basingstoke, Hampshire, England) at 25˚C for 24 h and the broth cultures were centrifuged at 5000 g at 4˚C for 15 min. The supernatant fluids were removed and the bacterial pellets were washed twice in phosphate buffered saline (PBS; 0.1 M, pH 7.2), then the pellets were collected in PBS as a stock bacterial suspension. The concentration of the culture was adjusted to an optical density of 1.39 at 520 nm using a spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan) to give a V. anguillarum concentration of 1010 colony forming units (CFU)/mL. The bacterial concentrations were confirmed by plate-counting on tryptone soya agar (Oxoid, Basingstoke, Hampshire, England).
To determine the LC50 (concentration lethal to 50% of test fish) to use in the experimental challenge, groups of ten yellowtail kingfish (51.98 ± 3.94 g, mean ± SD) were immersed in seawater (35 ppt) containing suspensions of V. anguillarum at 104, 105, 106, 107, 108 or 109 CFU/mL, or without addition of V. anguillarum (control) for 1 min. No mortality was observed in the control or in the bacterial suspensions of 104, 105 or 106 CFU/mL after 2 weeks. For 107, 108 and 109 CFU/mL, mortalities during 2 weeks after the immersion challenge were 10, 50 and 90%, respectively. The LC50 determined by extrapolation from the probit analysis as described by Finney [10] was 108 CFU/mL, which was used to challenge the experimental fish.
At the end of the growth trial, the fish were challenged by immersion in V. anguillarum suspension of 108 CFU/mL for 1 min. All the challenged fish were returned to their respective rearing tanks and fed twice daily for a further 4 weeks with the same experimental diet that was assigned before the challenge. Mortalities were recorded daily and dead fish were removed.
Necropsies of freshly dead fish from the lethal test and the bacterial challenged test were aseptically performed. The kidney and liver tissues were cultured to confirm death as a result of infection with V. anguillarum based on biochemical test methods of Buller [11].
2.4. Sample Collection
At the end of the growth trial and the end of the bacterial challenge, blood was sampled from the caudal vein of three fish/tank and directly used for measurement of haematocrit. The remaining whole blood was allowed to clot for 2 h at 4˚C and serum was separated for agglutinating antibody titer, lysozyme and bactericidal activity assays. The red blood cell pellets were used for glutathione peroxidase assay. Serum and red blood cell pellet samples were kept at −80˚C until analysis. Spleens (collected at the end of the challenge) and left anterior dorsal muscles from the sampled fish were dissected out and fixed in 10% buffered formalin for histological examination. The remaining muscles were filleted and used to determine Se contents.
2.5. Chemical Analysis
Gross energy was determined using a bomb calorimeter (C2000, IKA, Staufen, Germany). Protein, lipid, dry matter, ash and Se were determined according to the standard methods of the Association of Official Analytical Chemists [12]: crude protein by analysis of nitrogen using the Kjeldahl method; crude lipid by petroleum ether extraction using the Soxhlet method; dry matter by drying at 105˚C to a constant weight and ash by combustion at 550˚C for 24 h. Se was estimated using an atomic absorption spectrometer equipped with vapour generation assembly (AA280 FS and VGA 77, Varian, Mulgrave, VIC, Australia).
2.6. Survival and Growth Measurements
Mortality and the amount of feed eaten were recorded daily to calculate survival and feed intake, respectively. Fish in each tank were group weighed at the end of the growth trial to estimate weight gain. Weight measurement and feed intake were used for estimation of feed conversion ratio (FCR, feed intake divided by the wet weight gain).
2.7. Haematocrit
Haematocrit of each fish was determined in triplicate by the microhaematocrit method [13]. Blood was collected into heparin-coated microhaematocrit tubes and centrifuged at 13,000 g for 5 min to determine haematocrit (the percent packed cell volume).
2.8. Lysozyme Assay
Lysozyme activity was measured using the turbidimetric assay as described previously [14]. Briefly, 50 µL of serum was pipetted, in duplicate, in a 96-well plate (Iwaki, Tokyo, Japan). To each well was added 50 µL of Micrococcus lysodeiktikus (Sigma-Aldrich, St. Louis, MO, USA) suspended in PBS (0.25 mg/mL). The plate was monitored for absorbance at 450 nm every 2 min for a total of 20 min with a MS212 reader (Titertek Plus, Tecan, Austria). One unit of lysozyme activity was defined as the amount of enzyme resulting in a decrease in absorbance of 0.001/min.
2.9. Bactericidal Activity
Bactericidal activity was determined according to the method of Ueda et al. [15]. Fifty µL suspension of V. anguillarum in PBS (1.56 × 104 CFU/mL) was added to 50 µL tested serum, and the mixture was reacted for 30 min at 25˚C. The same volume of bacterial suspension was added to 50 µL of PBS as control, and was also reacted for 30 min at 25˚C simultaneously. After reaction 50 µL from the mixture was plated onto duplicate tryptone soya agar and incubated for 24 h at 25˚C. Bacterial activity was calculated as decrease in number of viable V. anguillarum cells, i.e. log10 CFU/mL in the control minus log10 CFU/mL in serum.
2.10. Glutathione Peroxidase Assay
Glutathione peroxidase (GPx) activity in red blood cells was assayed using the Ransel RS-505 kit (Randox, Crumlin, County Antrim, UK) and a chemistry immune analyser (AU400, Olympus, Tokyo, Japan). The results were expressed as units of GPx/g of haemoglobin (Hb). Haemoglobin was measured using the Hb HG-1539 kit (Randox, Crumlin, County Antrim, UK).
2.11. Serum Anti-V. anguillarum Antibody Titer
V. anguillarum was grown in tryptone soya broth at 25˚C for 24 h and killed in 1% formalin. The cells were centrifuged at 5000 g for 15 min at 4˚C. The resulting cell pellets were washed twice in PBS and suspended in PBS to an optical density of 0.151 at 520 nm (UV-1201 spectrophotometer, Shimadzu, Kyoto, Japan) and used as the antigen. Serum agglutinating antibody titer to V. anguillarum was determined with the serum agglutination technique described by Chen and Light [16] and reported as the last serum dilution which caused clumping of the antigen and transformed to log10 values for statistical analysis.
2.12. Histological Examination
The histological samples were routinely processed, dehydrated in ethanol before equilibration in xylene and embedded in paraffin wax. Sections of approximately 5 µm were cut and stained with haematoxylin and eosin or Perl’s Prussian blue [17] and observed under a light microscope (BX40F4, Olympus, Tokyo, Japan). Numbers of macrophage aggregates (MAs) per sections of entire spleens were assessed.
2.13. Data Analysis
Data were analysed using PASW Statistics 18.0 (IBM Corporation, New York, US). All data were subjected to a one-way ANOVA. Data were tested for normality and homogeneity of variance using Shapiro-Wilk and Levene’s tests, respectively. Where necessary, data were transformed to satisfy the assumptions of ANOVA. All percentage data were arcsine transformed prior to analysis. When a significant treatment effect was observed, Tukey’s Honest Significant Difference test was used for multiple mean comparisons. The statistical significance was set at P < 0.05 and the results were presented as means ± SE. Second-order regression analysis [18] was performed on weight gain vs. Se concentrations in diets to estimate dietary Se requirement for yellowtail kingfish.
3. RESULTS
During 6 weeks of feeding, dietary Se did not influence feed intake, FCR and survival of the fish, which remained 100% (Table 1). However, weight gain was significantly (P < 0.05) affected by the dietary treatments (Table 1), the fish fed the control diet gained significantly (P < 0.05) less weight than fish fed the supplemented Se diets, which produced similar weight gains. Second order regression analysis of the levels of Se in diet vs. weight gain (Figure 1) indicated the optimal Se level for maximal growth of yellowtail kingfish was 5.56 mg/kg (y = −1.5579x2 + 17.328x − 5.039, R2 = 0.989). When the relationship between the supplementation levels of Se and weight gain data were analysed by polynomial regression, the maximum of the curve obtained was 2.19 mg/kg (y = −1.5935x2 + 6.9733x + 35.523, R2 = 0.9897, figure not shown).
Se supplementation had no significant effect on haema-
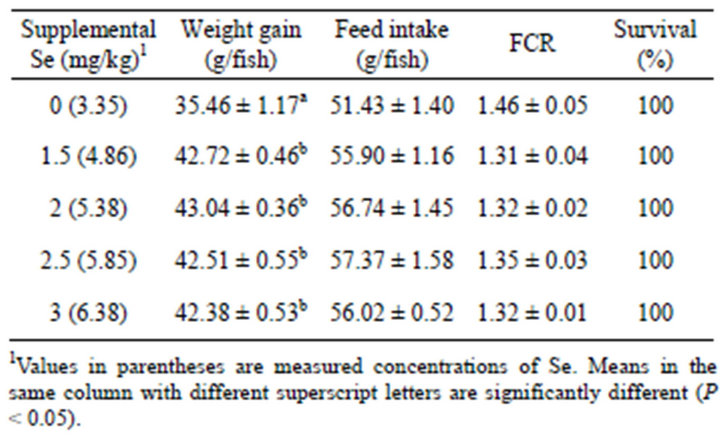
Table 1. Mean ± SE weight gain, feed intake, feed conversion ratio (FCR) and survival of yellowtail kingfish fed diets containing various inclusion levels of Se for 6 weeks.
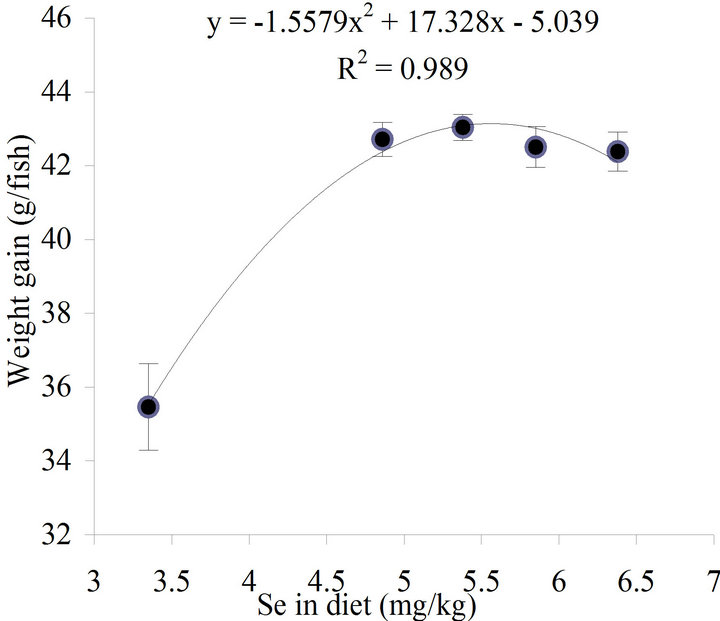
Figure 1. Relationship between concentration of dietary Se and weight gain of yellowtail kingfish fed the experimental diets for 6 weeks. The optimal Se level for maximal growth of yellowtail kingfish derived from second order regression method was 5.56 mg/kg.
tocrit and lysozyme activity of the pre-challenged fish, but affected the post-challenged fish (Table 2). During post-challenge period, the haematocrit and lysozyme activity were significantly higher (P < 0.05) in the fish fed Se supplemented diets than the fish fed the diet without supplementation. In both preand post-challenged fish, bactericidal and glutathione peroxidase activities were stimulated by Se supplements, fish fed Se supplemented diets had significantly higher (P < 0.05) bactericidal and glutathione peroxidase activities than fish fed the un-supplemented diet, while the increases in Se intake by the fish resulted in significant increases (P < 0.05) of Se concentrations in fillets (Table 2).
As a result of the bacterial challenge, lysozyme, bactericidal and glutathione peroxidase activities were significantly elevated (P < 0.05). In contrast, haematocrit and Se in fillets decreased significantly (P < 0.05) by the challenge (Table 2).
The bacterial infection resulted in significantly higher mortalities in control-diet fed-fish than fish fed the diets supplemented with Se at more than 1.5 mg/kg (Table 3). Antibody titers against V. anguillarum were significantly increased (P < 0.05) with dietary Se supplementation of 2 mg/kg or more in comparison with the antibody titer of the control group (Table 3).
After the bacterial challenge, the surviving fish fed the un-supplemented diet had significantly higher (P < 0.05) numbers of macrophage aggregates (MAs) in spleen than the fish fed the Se-supplemented diets (Table 3). Within a MA, melanin, haemosiderin and lipofuscin/ceroid were clearly seen by the Perl’s Prussian blue stain (Figure 2). The necrotic fibres in muscle were observed in the fish fed the control diet (Figure 3), but not in the fish fed the other diets.
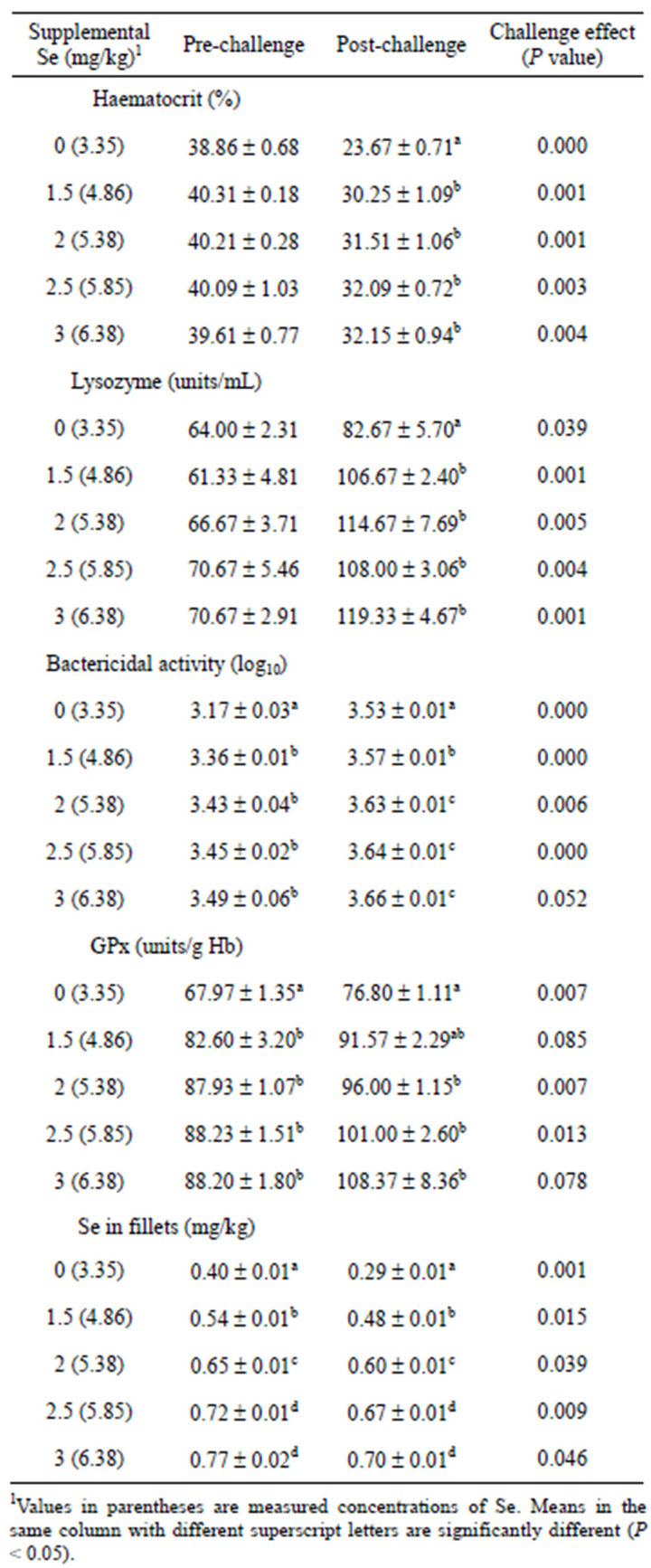
Table 2. Mean ± SE haematocrit, lysozyme, bactericidal and glutathione peroxidase (GPx) activities, and Se in fillets of yellowtail kingfish fed diets containing various inclusion levels of Se and subsequently challenged with V. anguillarum.
4. DISCUSSION
The biosynthesis of selenoproteins is primarily depend-
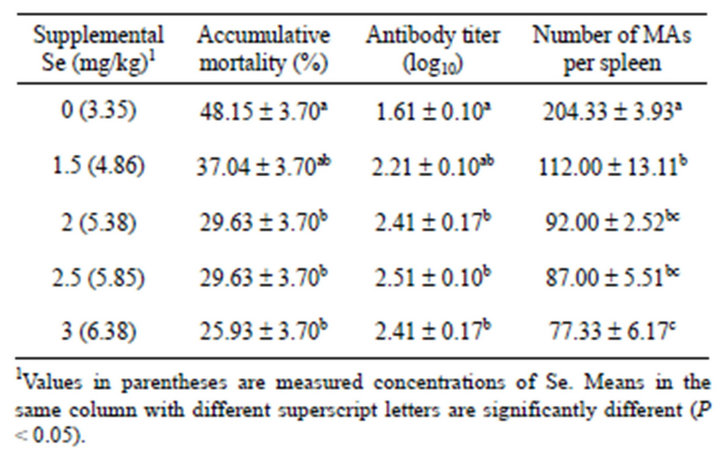
Table 3. Mean ± SE accumulative mortality, antibody to V. anguillarum and number of macrophage aggregates (MAs) in spleen of yellowtail kingfish fed diets containing various inclusion levels of Se and subsequently challenged with V. anguillarum.
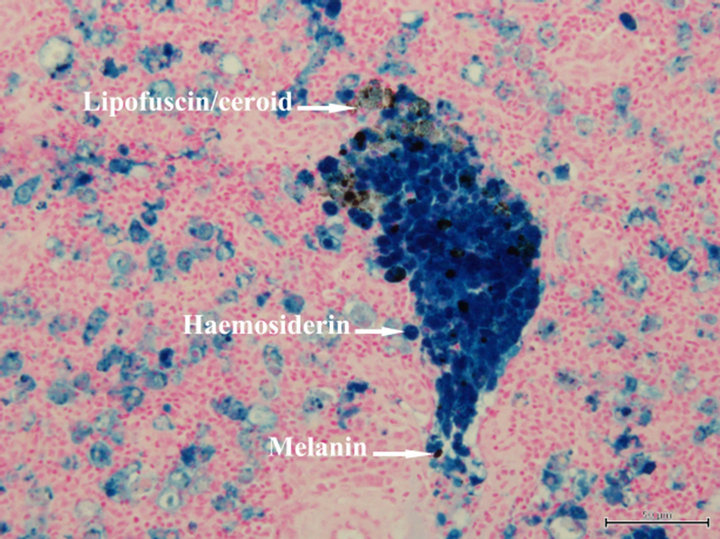
Figure 2. A macrophage aggregate in a section of spleen of yellowtail kingfish fed the control diet and subsequently challenged with V. anguillarum. Perl’s Prussian blue stain, scale bar = 50 µm.
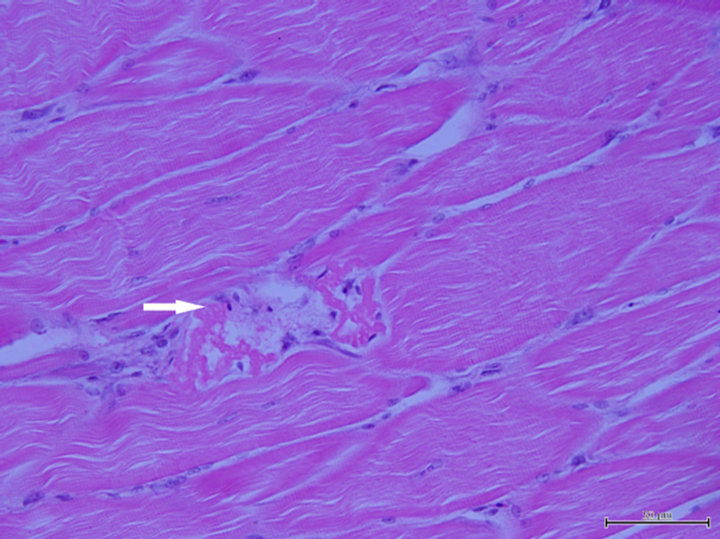
Figure 3. Section of muscle of yellowtail kingfish fed the control diet, showing necrotic fibres (arrow). Haematoxylin and eosin stain, scale bar = 50 µm.
ent on Se supply and consequently on the formation of selenocysteine-specific tRNA, which regulates the level of all selenoproteins [19]. In poultry, Se supplementation has been reported to increase expression of genes involved in energy production and protein synthesis pathways [20]. Supplementation of Se may increase growthrelated gene expression in yellowtail kingfish. In the present study, the beneficial growth effect of dietary Se for yellowtail kingfish was shown by the weight gain data and this is consistent with the data reported for grouper (Epinephelus malabaricus) [21], cobia (Rachycentron canadum) [22] and gibel carp (Carassius auratus gibelio) [23]. The optimal Se requirement for maximal growth of yellowtail kingfish obtained from our study is higher than that reported for other fish species [21-23]. Yellowtail kingfish is very active and fast growing and hence may require high Se input.
Bacterial challenge has been used as a final test to evaluate relationships between nutrients and immune responses of fish [24]. The results of bacterial challenge in the present study showed that dietary Se improved immune responses and resistance of yellowtail kingfish to V. anguillarum infection. Supplementation of Se at ≥2 mg/kg (measured Se of 5.38 mg/kg) significantly increased survival following infection with V. anguillarum and there was a corresponding increase in antibody titer. The same effects of Se on survival and antibody response have been reported for channel catfish challenged with pathogenic bacterium Edwardsiella ictaluri [25]. The immune-stimulating effects of dietary Se were also demonstrated in bactericidal and lysozyme activities. Serum of yellowtail kingfish had the ability to inhibit the growth of V. anguillarum and this ability was stimulated by dietary Se, while lysozyme activity in serum of postchallenged fish increased with an increase of Se in diets. Supplementation of Se promotes lymphocyte protein synthesis and may lead to increased immune cell activity and thus results in an improved immune capacity [26]. Furthermore, there were significant increases in glutathione peroxidase activities in response to Se supplementation in both preand post-challenged fish. Glutathione peroxidase is one of the most important antioxidant defence enzymes in fish [27] and its activity is dependent on the dietary Se intake. The glutathione peroxidase activity was shown to decrease in Atlantic salmon (Salmo salar) [28] and channel catfish (Ictalurus punctatus) [29] fed diets deficient in Se, whereas antioxidant capacity of grouper [21] and cobia [22] measured as glutathione peroxidase activity increased as dietary Se increased.
The V. anguillarum infection caused haematological changes which included a significant decrease in haematocrit, and the presence of abundant macrophage aggregates in spleens of yellowtail kingfish. The haematocrit of the fish fed the control diet decreased to 23.67%, lower than haematocrit of Japanese yellowtail (Seriola quinqueradiata) considered in an anaemic state, 27.00% [30]. The decrease in haematocrit has also been found in coho salmon (Oncorhynchus kisutch) [31] and rainbow trout (Oncorhynchus mykiss) [32] infected with V. anguillarum. Haemolysin produced by V. anguillarum is believed to be responsible for the haemolytic anaemia in infected fish [33]. The decreases in haematocrits and Se content in fillets and the increases in glutathione peroxidase activities as a result of bacterial infection indicate an increased requirement for Se under infected conditions.
The spleen macrophage aggregates play an important role in the storage of damaged cells including red blood cells and they change in number in relation to fish health, and fish in poor health or nutritionally deprived tend to have more macrophage aggregates [34]. Thus, they have been suggested as reliable cytological biomarkers for assessment of fish health. In the present study, more macrophage aggregates were observed in the fish fed the control diet deficient in Se than in those fed Se supplemented diets, indicating improved physiological condition of fish being fed Se supplementation. In addition, muscle necrosis observed in the present study confirms the necessity of supplementation of Se for prevention of myopathy in yellowtail kingfish.
On the basis of the results of this study, it can be concluded that a diet containing 5.56 mg/kg Se was the optimal level for maximal growth and satisfied the requirement under susceptible condition for yellowtail kingfish.
5. ACKNOWLEDGEMENTS
This research was sponsored by Endeavour Awards. The authors wish to acknowledge the assistance of Dr Fran Stephens for her technical assistance with histology.
REFERENCES
- Booth, M.A., Allan, G.L. and Pirozzi, I. (2010) Estimation of digestible protein and energy requirements of yellowtail kingfish Seriola lalandi using a factorial approach. Aquaculture, 307, 247-259. doi:10.1016/j.aquaculture.2010.07.019
- Bowyer, J.N., Qin, J.G., Smullen, R.P. and Stone, D.A.J. (2012) Replacement of fish oil by poultry oil and canola oil in yellowtail kingfish (Seriola lalandi) at optimal and suboptimal temperatures. Aquaculture, 356-357, 211-222. doi:10.1016/j.aquaculture.2012.05.014
- National Research Council (1993) Nutrient requirements of fish. National Academy Press, Washington DC.
- Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G. and Hoekstra, W.G. (1973) Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588-590. doi:10.1126/science.179.4073.588
- Kiremidjian-Schumacher, L. and Stotzky, G. (1987) Selenium and immune responses. Environmental Research, 42, 277-303. doi:10.1016/s0013-9351(87)80194-9
- Arthur, J.R., McKenzie, R.C. and Beckett, G.J. (2003) Selenium in the immune system. The Journal of Nutrition, 133, 1457s-1459s.
- Colwell, R.R. and Grimes, D.J. (1984) Vibrio diseases of marine fish populations. Helgolander Meeresunters, 37, 265-287. doi:10.1007/BF01989311
- Pedersen, K. and Larsen, J.L. (1993) rRNA gene restriction patterns of Vibrio anguillarum serogroup O1. Diseases of Aquatic Organisms, 16, 121-126. doi:10.3354/dao016121
- Webster, C.D. and Lim, C.E., Eds. (2002) Nutrient requirements and feeding of finfish for aquaculture. CABI Publishing, Wallingford, Oxon.
- Finney, D.J. (1971) Probit analysis. 3rd Edition, Cambridge University Press, Cambridge.
- Buller, N.B. (2004) Bacteria from fish and other aquatic animals: A practical identification manual. CABI Publishing, Oxfordshire.
- Association of Official Analytical Chemists (1990) Official methods of analysis of the Association of Official Analytical Chemists. 15th Edition, The Association of Official Analytical Chemists, Arlington.
- Rey Vázquez, G. and Guerrero, G.A. (2007) Characterization of blood cells and hematological parameters in Cichlasoma dimerus (Teleostei, Perciformes). Tissue and Cell, 39, 151-160. doi:10.1016/j.tice.2007.02.004
- Bowden, T.J., Butler, R. and Bricknell, I.R. (2004) Seasonal variation of serum lysozyme levels in Atlantic halibut (Hippoglossus hippoglossus L.). Fish & Shellfish Immunology, 17, 129-135. doi:10.1016/j.fsi.2003.12.001
- Ueda, R., Sugita, H. and Deguchi, Y. (1999) Effect of transportation on the serum bactericidal activity of Penaeus japonicus and Ovalipes punctatus. Aquaculture, 171, 221- 225. doi:10.1016/s0044-8486(98)00492-x
- Chen, M.F. and Light, T.S. (1994) Communications: Specificity of the channel catfish antibody to Edwardsiella ictaluri. Journal of Aquatic Animal Health, 6, 266- 270. doi:10.1577/1548-8667(1994)006<0266:csotcc>2.3.co;2
- Luna, L.G. (1968) Manual of histologic staining methods of the Armed Forces Institute of Pathology. 3rd Edition, McGraw-Hill, New York.
- Shearer (2000) Experimental design, statistical analysis and modelling of dietary nutrient requirement studies for fish: A critical review. Aquaculture Nutrition, 6, 91-102. doi:10.1046/j.1365-2095.2000.00134.x
- Fischer, A. and Pallauf, J. (2005) Dietary selenium and gene expression. In: Rimbach, G., Fuchs, J. and Packer, L., Eds., Nutrigenomics, Taylor & Francis Group, New York, 441-455.
- Brennan, K.M., Crowdus, C.A., Cantor, A.H., Pescatore, A.J., Barger, J.L., Horgan, K., Xiao, R., Power, R.F. and Dawson, K.A. (2011) Effects of organic and inorganic dietary selenium supplementation on gene expression profiles in oviduct tissue from broiler-breeder hens. Animal Reproduction Science, 125, 180-188. doi:10.1016/j.anireprosci.2011.02.027
- Lin, Y.-H. and Shiau, S.-Y. (2005) Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture, 250, 356-363. doi:10.1016/j.aquaculture.2005.03.022
- Liu, K., Wang, X.J., Ai, Q., Mai, K. and Zhang, W. (2010) Dietary selenium requirement for juvenile cobia, Rachycentron canadum L. Aquaculture Research, 41, e594-e601. doi:10.1111/j.1365-2109.2010.02562.x
- Han, D., Xie, S., Liu, M., Xiao, X., Liu, H., Zhu, X. and Yang, Y. (2011) The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquaculture Nutrition, 17, e741-e749. doi:10.1111/j.1365-2095.2010.00841.x
- Landolt, M.L. (1989) The relationship between diet and the immune response of fish. Aquaculture, 79, 193-206. doi:10.1016/0044-8486(89)90461-4
- Wang, C., Lovell, R.T. and Klesius, P.H. (1997) Response to Edwardsiella ictaluri challenge by channel catfish fed organic and inorganic sources of selenium. Journal of Aquatic Animal Health, 9, 172-179. doi:10.1577/1548-8667(1997)009<0172:rteicb>2.3.co;2
- Pagmantidis, V., Méplan, C., Schothorst, E.M.V., Keijer, J. and Hesketh, J.E. (2008) Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. The American Journal of Clinical Nutrition, 87, 181-189.
- Ross, S.W., Dalton, D.A., Kramer, S. and Christensen, B.L. (2001) Physiological (antioxidant) responses of estuarine fishes to variability in dissolved oxygen. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 130, 289-303. doi:10.1016/s1532-0456(01)00243-5
- Bell, J.G., Cowey, C.B., Adron, J.W. and Pirie, B.J.S. (1987) Some effects of selenium deficiency on enzyme activities and indices of tissue peroxidation in Atlantic salmon parr (Salmo salar). Aquaculture, 65, 43-54. doi:10.1016/0044-8486(87)90269-9
- Wise, D.J., Tomasso, J.R., Gatlin, D.M., Bai, S.C. and Blazer, V.S. (1993) Effects of dietary selenium and vitamin E on red blood cell peroxidation, glutathione peroxidase activity, and macrophage superoxide anion production in channel catfish. Journal of Aquatic Animal Health, 5, 177-182. doi:10.1577/1548-8667(1993)005<0177:eodsav>2.3.co;2
- Watanabe, T., Aoki, H., Shimamoto, K., Hadzuma, M., Maita, M., Yamagata, Y., Kiron, V. and Satoh, S. (1998) A trial to culture yellowtail with non-fishmeal diets. Fisheries Science, 64, 505-512.
- Harbell, S.C., Hodgins, H.O. and Schiewe, M.H. (1979) Studies on the pathogenesis of vibriosis in coho salmon Oncorhynchus kisutch (Walbaum). Journal of Fish Diseases, 2, 391-404. doi:10.1111/j.1365-2761.1979.tb00391.x
- Lamas, J., Santos, Y., Bruno, D., Toranzo, A.E. and Anadon, R. (1994) A comparison of pathological changes caused by Vibrio anguillarum and its extracellular products in rainbow trout (Oncorhynchus mykiss). Fish Pathology, 29, 79-89. doi:10.3147/jsfp.29.79
- Munn, C.B. (1978) Haemolysin production by Vibrio anguillarum. FEMS Microbiology Letters, 3, 265-268. doi:10.1111/j.1574-6968.1978.tb01944.x
- Wolke, R.E. (1992) Piscine macrophage aggregates: A review. Annual Review of Fish Diseases, 2, 91-108. doi:10.1016/0959-8030(92)90058-6

