Advances in Infectious Diseases
Vol.4 No.3(2014), Article ID:47645,7 pages
DOI:10.4236/aid.2014.43018
Clinical and Pathological Comparison of Pyogenic and Amoebic Liver Abscesses
Adnan Bashir Bhatti*,Farhan Ali, Siddique A. Satti, Tariq M. Satti
Capital Development Authority (CDA) Hospital, Islamabad, Pakistan
Email: *dr.adnanbashir@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 January 2014; revised 26 February 2014; accepted 26 March 2014
ABSTRACT
BACKGROUND: Pyogenic and amoebic liver abscesses are rare, potentially lethal conditions. In this study, we aimed to examine the clinical and pathological differences between them. METHODS: Patients with confirmed liver abscesses were divided into two groups: the pyogenic (n = 47) and amoebic group (n = 21), which were analyzed for differences in clinical and laboratory findings. RESULTS: Amoebic liver abscesses presented most frequently in young adults (14 - 30 years; 71%), whereas pyogenic liver abscesses were most commonly observed in adults 41 - 50 years (49%). Indirect hemagglutination test revealed a 100% positive response in the amoebic group, whereas 68% of the pyogenic group presented with blood/pus culture. Multiple abscesses were observed in 66% and 24% of patients in the pyogenic and amoebic group, respectively. CONCLUSIONS: Pyogenic abscesses were commonly observed in older patients, and were associated with features such as markedly deranged liver function test, higher prothrombin time, and multiple abscesses, compared to amoebic abscess. Early and improved diagnoses and differentiation between the two conditions, followed by the correct treatment, can help prevent serious complications and lead to an overall improved mortality rate.
Keywords:Amoebic liver Abscess, Pyogenic Liver Abscess, Entamoeba histolytica, Leukocytosis

1. Introduction
Liver abscesses have been recognized for centuries, and in 1883, amoebae were first described as a cause of liver abscesses. In 1938, the largest series of pyogenic and amoebic liver abscesses in the literature for the time was published [1] , and despite a refinement in diagnostic and therapeutic modalities since then, liver abscess remains a serious condition with a high morbidity and mortality rate [1] -[3] .
Worldwide, approximately 40 - 50 million people are infected annually with amoebic abscesses, with the majority of infections occurring in developing countries. In endemic areas, the prevalence of infection is higher than 5% - 10% [3] , and has been reported to be as high as 55% in certain areas [4] . These areas include, but are not limited to, rural areas of Central and South America, India, and the tropical areas of Asia and Africa [5] . Conversely, the incidence of pyogenic liver abscess has remained more or less unchanged since the 1950’s. In the United States, the incidence of pyogenic liver abscess is estimated to be 8 - 15 cases per 100,000, but this figure is considerably higher in countries where health care is not readily available [6] .
Amoebic liver abscesses are caused by E. histolytica. E. histolytica exists in 2 forms: the cyst stage, which is the infective form, and the trophozoite stage, which causes the invasive disease. Invasion by E. histolytica into mesenteric venules can result in the amoebae entering the portal circulation and travel to the liver where they typically form large abscesses [7] . Pyogenic abscesses of the liver often occur secondary to infections of the adjacent tissues or biliaryor intestinal tracts or hematogenous seeding, and are associated with a mortality rate of 20% - 60%, even with appropriate medical-surgical management [2] [3] . The signs and symptoms of amoebic liver abscess are often relatively non-specific, and resemble those associated with pyogenic liver abscesses or other febrile diseases [5] [8] -[10] .
The rationale of this study was to explore the differences between clinical presentations and literature findings, for pyogenic and amoebic liver abscesses. By conducting this study we were able to suggest possible improvements in the management plan of these disorders, and we conclude that correct diagnosis and timely intervention can improve the overall outcomes for these patients.
2. Materials and Methods
2.1. Overview
This study was conducted in the emergency and outpatient medical department of Capital Hospital Islamabad, Pakistan, which is a tertiary care hospital, from January 1, 2010 to December 31, 2012. After receiving permission from the concerned authorities of Capital Hospital Islamabad (Head of the Department of Medicine, Hospital Ethical Committee), the data collection phase was started on January 1, 2010.
2.2. Data Collection
By non-probability convenient sampling, 68 patients with confirmed pyogenic or amoebic liver abscesses aged 14 years or older were included in this study.
Before commencing the study, verbal consent was taken from all the patients after explaining the nature and purpose of the study. All patients were handled by the same doctor to minimize bias. Patients with pyogenic and amoebic liver abscesses were identified, and detailed history was taken for diagnosis and fulfillment of the required selection criteria.
Using structured proforma, information was collected. Patient age and sex was noted, and by vigorous and strict criteria only patients of confirmed pyogenic and amoebic liver abscesses were selected for the study. Patients were divided into amoebic group and pyogenic groups, and detailed medical histories were taken regarding symptoms or conditions such as fever, right hypochondrium pain, pruritis, diarrhea, shortness of breath, weight loss and anorexia.
2.3. Methods
General physical examination was done for jaundice, skin rashes and vitals such as blood pressure, temperature, pulse and respiratory rates. A detailed, complete systemic examination was moreover performed for tender hepatomegaly, ascities and pleural effusion, as well as for other systemic symptoms.
Patient samples were examined using a number of tests, including blood complete picture, erythrocyte sedimentation rate (ESR), blood cultures, liver functions test, serum albumin, prothrombin time, chest X-rays for pleural effusion, ultrasound of the abdomen, and computed tomography (CT) scan of the abdomen, to look for the number and size of abscesses. Liver abscesses were aspirated to examine the type of abscess, and amoebic liver abscess was confirmed and diagnosed by using the indirect hemagglutination test.
2.4. Data Analysis Procedure
The statistical package for social sciences (SPSS, version 13.0, Chicago, IL) was used to analyze the data. Means, frequencies and percentages were calculated and chi square test was used to determine the significance. For this study, a P-value of <0.05 was considered as being significant.
2.5. Exclusion Criteria
Patients diagnosed with Malaria, Enteric fever, pyrexia of unknown origin, Acute cholecystitis, acute viral hepatitis (A, B, or E), chronic liver diseases, or hepatomegaly due to any cause, were excluded from the study.
3. Results
We analyzed the clinical characteristics of 68 patients of liver abscesses. 47 were diagnosed with pyogenic liver abscesses, and 21 were diagnosed with amoebic liver abscessed. For both groups, the majority of patients were male (Figure 1). In the pyogenic group, the majority of patients were between 41 and 50 years old, while in the amoebic group, most patients were between 14 and 30 years old (Table 1).
Fever, jaundice, and right hypochondrium pain were the main clinical features for both groups (Table 2) Multiple abscess formation was more common in the pyogenic group (66%), while a single abscess was more common in the amoebic group (76%), as depicted in Figure 2.

Figure 1. Sex of patients in pyogenic and amoebic groups.
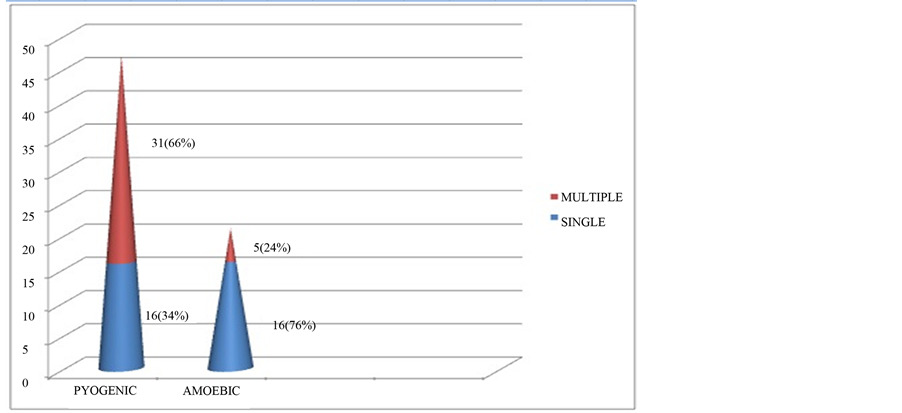
Figure 2. Number of abscesses in pyogenic and amoebic groups.
Table 1. Pyogenic and amoebic liver abscess patient age.
Table 2. Clinical features of pyogenic and amoebic liver abscesses.
For the pyogenic group, 32 patients (68%) had right lobe involvement, whereas this number 17 (81%) for the amoebic group. Total leukocyte count ranged between 15 - 22 × 109/L in the pyogenic group, and between 11 - 16 × 109/L in the amoebic group. ESR was high in both groups; 60 - 90 mm in 1 hour in the pyogenic group, and between 40 - 55 mm in 1 hour in the amoebic group. Alanine aminotransferase (ALT) was between 200 - 250 u/l in pyogenic group, and between 80 - 110 u/l in the amoebic group. The value of Aspartate aminotransferase (AST) was between 160 - 230 u/L in the pyogenic group, and between 50 - 120 u/L in the amoebic group. The value of serum bilirubin was high; between 2.9 - 3.4 mg/dl in the pyogenic group and between 1.2 - 2.4 mg/dl in the amoebic group. Prothrombin time was between 25 - 35 sec in the pyogenic group and between 15 - 20 sec in amoebic group. These findings are summarized in Table3
Ultrasound guided aspirations were done thrice in 17 (36.1%) cases of pyogenic group, and in only 1 (4.7%) case of amoebic abscess as listed in Table4 In the pyogenic abscess group, green or yellow aspirate was observed, whereas for amoebic abscesses, the aspirate was brown.
4. Discussion
Hepatic abscesses are divided into three categories: pyogenic, amoebic and fungal, all which are associated with a number of disease processes. The liver receives blood from both systemic and portal circulations, and hence a high level of susceptibility to infection is expected, given the enhanced exposure to bacteria. Pyogenic liver abscesses are frequently reported to be polymicrobial; amoebic abscesses are mainly due to Entamoeba histolytica; and fungal abscesses are reported to be due to a variety of candida species [11] [12] . In our study we evaluated the clinical manifestations of, and differences between, amoebic and pyogenic abscesses.
Over two decades ago, Barnes et al. showed that amoebic abscesses were common in younger patients, while pyogenic abscesses were more common in older patients [13] . Greenstein et al. also showed that the incidence of male patients was greater compared to female patients in both types of liver abscesses, and that multiple abscesses were common in pyogenic liver abscesses but not amoebic [14] , and our study here showed very similar results.
Another study from 2004 reported that fever, right-upper-quadrant abdominal pain, and hepatomegaly were major clinical features for both types of hepatic abscesses, while jaundice was commonly observed in pyogenic abscesses only [15] . Conter et al. showed that spiking fever with chills, jaundice, septic shock, palpable mass, and right hypochondrium pain were all features of pyogenic abscesses, while patients with amoebic liver abscesses were more likely to present with abdominal pain, diarrhea, and hepatomegaly [16] . We observed very similar results in this present study, with the exception that no patient developed septic shock, and only a few cases of pleural effusions were present in pyogenic group.
While one study has previously showed that the right lobe of the liver was the most likely site of infection in both types of hepatic abscess [15] , another study showed that leukocytosis, decreased serum albumin, raised ALT, and AST were more marked in pyogenic abscess as compared to amoebic abscesses [16] , and these results also correspond with the results from our study. In addition, we also detected a raised prothrombin time in the pyogenic abscess group.
CT scans and ultrasonography have already been established as excellent methods of detecting the presence, but not the type, of liver abscess [16] . Ultrasound is usually the method of choice for most physicians, and is generally capable of detecting up to 100% of all abscesses. The indirect hemagglutination test has been shown to be 100% accurate for the confirmation of amoebic liver abscess, with up to 90% of pyogenic abscesses showing positive blood and pus cultures [17] . In our study, we made our diagnoses using a combination of ultrasonography, indirect hemagglutination tests and blood/pus cultures. In a few cases, CT scans were also done to reach a final, confident diagnosis.
Untreated hepatic abscesses are nearly almost fatal, due to the numerous complications that can arise. Treatment traditionally include drainage, either percutaneous or surgical, and antibiotic parenteral antibiotic therapy, with a recommended duration of 2 - 3 weeks, or until there is a favorable clinical response. Complementary oral antimicrobial therapy is then administered for a further 2 - 4 weeks or a complete resolution of the abscess cavity can be demonstrated [11] [18] [19] .
Evidence suggests that antibiotic therapy alone is usually not sufficient to entirely resolve a liver abscess unless it is <3 cm, and it has been suggested that abscesses larger than 3 cm should be routinely drained [19] . With the refinement of image-guided techniques in recent years, percutaneous drainage and aspiration, under CT or ultrasound guidance, have emerged as appropriate alternatives to open drainage, and are providing success rates comparable to those of traditional methods, while being minimally invasive [20] -[22] . In this study, we treated our cases with broad-spectrum amoebicidal antibiotics, and abscesses larger than 3 cm were furthermore treated with ultrasound-guided percutaneous aspiration after correction of coagulopathy with transfusion of fresh frozen plasma prior to drainage. We monitored the size of the abscesses using ultrasonography, and performed two or even three percutaneous aspirations in some patients.
5. Conclusion
Hepatic abscesses are a relatively rare occurrence, but an important problem nonetheless. Improved awareness can decrease complications and mortality rates. Clinical features of pyogenic abscesses and amoebic abscesses are somewhat similar, but pyogenic abscesses are generally described as being more aggressive. Ultrasound and CT scans play key roles in diagnosis, but confirmation using blood/pus cultures and indirect hemagglutination tests are also recommended. Early treatment with broad-spectrum amoebicidal antibiotics, and percutaneous aspiration can prevent serious complications.
References
- Ochsner, A., DeBakey, M. and Murray, S. (1938) Pyogenic Abscess of the Liver: II. An Analysis of Forty-Seven Cases with Review of the Literature. The American Journal of Surgery, 40, 292-319. http://dx.doi.org/10.1016/S0002-9610(38)90618-X
- Blessmann, J., Ali, I.K., Ton Nu, P.A., Dihn, B.T., et al. (2003) Longitudinal Study of Intestinal Entamoeba histolytica Infections in Asymptomatic Adult Carriers. Journal of Clinical Microbiology, 41, 4745-4750. http://dx.doi.org/10.1128/JCM.41.10.4745-4750.2003
- Stanley Jr., S.L. (2003) Amoebiasis. The Lancet, 361, 1025-1034. http://dx.doi.org/10.1016/S0140-6736(03)12830-9
- Haque, R., Duggal, P., Ali,
I.M., Hossain, M.B., et al. (2002) Innate and Acquired Resistance to Amebiasis in
Bangladeshi Children. The Journal of Infectious Diseases, 186, 547-552.
http://dx.doi.org/10.1086/341566 - Ralston, K.S. and Petri Jr., W.A. (2011) Tissue Destruction and Invasion by Entamoeba histolytica. Trends in Parasitology, 27, 254-263. http://dx.doi.org/10.1016/j.pt.2011.02.006
- Branum, G.D., Tyson, G.S., Branum,
M.A. and Meyers, W.C. (1990) Hepatic Abscess. Changes in Etiology, Diagnosis, and
Management. Annals of Surgery, 212, 655-662.
http://dx.doi.org/10.1097/00000658-199012000-00002 - Blazquez, S., Rigothier, M.C., Huerre, M. and Guillen, N. (2007) Initiation of Inflammation and Cell Death during liver Abscess Formation by Entamoeba histolytica Depends on Activity of the Galactose/N-acetyl-D-galactosamine Lectin. International Journal for Parasitology, 37, 425-433. http://dx.doi.org/10.1016/j.ijpara.2006.10.008
- Gyorffy, E.J., Frey, C.F., Silva Jr., J. and McGahan, J. (1987) Pyogenic Liver Abscess. Diagnostic and Therapeutic Strategies. Annals of Surgery, 206, 699-705. http://dx.doi.org/10.1097/00000658-198712000-00003
- Hoffner, R.J., Kilaghbian, T., Esekogwu, V.I. and Henderson, S.O. (1999) Common Presentations of Amebic Liver Abscess. Annals of Emergency Medicine, 34, 351-355. http://dx.doi.org/10.1016/S0196-0644(99)70130-7
- Hughes, M.A. and Petri Jr., W.A. (2000) Amebic Liver Abscess. Infectious Disease Clinics of North America, 14, 565-582. http://dx.doi.org/10.1016/S0891-5520(05)70121-5
- Chen, S.C., Huang, C.C., Tsai, S.J., Yen, C.H., et al. (2009) Severity of Disease as Main Predictor for Mortality in Patients with Pyogenic Liver Abscess. The American Journal of Surgery, 198, 164-172. http://dx.doi.org/10.1016/j.amjsurg.2008.08.022
- Othman, N., Mohamed, Z., Yahya, M.M., Leow, V.M., et al. (2013) Entamoeba histolytica Antigenic Protein Detected in Pus Aspirates from Patients with Amoebic Liver Abscess. Experimental Parasitology, 134, 504-510. http://dx.doi.org/10.1016/j.exppara.2013.05.001
- Barnes, P.F., De Cock, K.M., Reynolds, T.N. and Ralls, P.W. (1987) Comparison of Amoebic and Pyogenic Abscess of the Liver. Medicine, 66, 472-483. http://dx.doi.org/10.1097/00005792-198711000-00005
- Greenstein, A.J., Barth, J., Dicker, A., Bottone, E.J., et al. (1985) Amoebic Liver Abscess: A Study of 11 Cases Compared with a Series of 38 Patients with Pyogenic Liver Abscess. Am J Gasteroenterol, 80, 472-478.
- Kurland, J.E. and Brann, O. (2004) Pyogenic and amoebic liver abscess. Current Gasteroenterology Reports, 6, 273-279. http://dx.doi.org/10.1007/s11894-004-0078-2
- Conter, R.L., Pitt, H.A., Tompkins, R.K. and Longmire Jr., W.P. (1986) Differentiation of Pyogenic from Amebic Hepatic Abscesses. Surgery, Gynecology & Obstetrics, 162, 114-120.
- Donovan, A.J., Yellin, A.E. and Ralls, P.W. (1991) Hepatic Abscess. World Journal of Surgery, 15, 162-169. http://dx.doi.org/10.1007/BF01659049
- Chung, Y.F., Tan, Y.M., Lui, H.F., Tay, K.H., et al. (2007) Management of Pyogenic Liver Abscesses—Percutaneous or Open Drainage. Singapore Medical Journal, 48, 1158-1165.
- Hope, W.W., Vrochides, D.V., Newcomb, W.L., Mayo-Smith, W.W., et al. (2008) Optimal Treatment of Hepatic Abscess. The American Surgeon, 74, 178-182.
- Giorgio, A., de Stefano, G., Di Sarno, A., Liorre, G., et al. (2006) Percutaneous Needle Aspiration of Multiple Pyogenic Abscesses of the Liver: 13-Year Single-Center Experience. American Journal of Roentgenology, 187, 1585-1590. http://dx.doi.org/10.2214/AJR.05.1104
- Men, S., Akhan, O. and Koroglu, M. (2002) Percutaneous Drainage of Abdominal Abcess. European Journal of Radiology, 43, 204-218. http://dx.doi.org/10.1016/S0720-048X(02)00156-0
- O’Farrell, N., Collins, C.G. and McEntee, G.P. (2010) Pyogenic Liver Abscesses: Diminished Role for Operative Treatment. The Surgeon, 8, 192-196. http://dx.doi.org/10.1016/j.surge.2010.01.001
NOTES

*Corresponding author.


