Journal of Materials Science and Chemical Engineering
Vol.05 No.04(2017), Article ID:75745,8 pages
10.4236/msce.2017.54001
Degumming of Silk Fibers by CO2 Supercritical Fluid
Chung-Haur Lo, Yuchou Chao
Institute of Organic and Polymeric Materials, National Taipei University of Technology, Taiwan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 10, 2017; Accepted: April 25, 2017; Published: April 28, 2017
ABSTRACT
This paper is to study a new method to remove sericin from raw silk fiber. This new process is done using an organic acid as a pretreatment and then using CO2 supercritical fluid to remove sericin from silk fiber. This method would be a huge break from the traditional environmentally unsustainable methods used today. This new processing method keeps the removed sericin in a clean state that can be used as a highly marketable silk protein in the medical and cosmetic industries.
Keywords:
Silk, Sericin, Degumming, CO2 Supercritical Fluid

1. Introduction
Silk fibers are spun as a natural process of silk worms. Silk (Bombyx Mori) is one of the world’s most revered natural fibers used by the fashion industry. This is due to it having exceptional characteristics such as: long fine fibers, high tensile strength, a rich luster and numerous mechanical properties.
Throughout history, silk has been valued greatly and has been used as the benchmark when creating synthetic fibers. Silk is known for its comfortable touch to the body, moisture regulation properties, staying cool in hot conditions, and excellent ventilation. Silks high-class luster has also increased the fibers status in the fashion world.
Recently, due to consumers increased awareness of the many environmental problems associated with the production of petro-chemical synthetic fibers, silk has attracted even more commercial attention. Silk fibers are considered as an eco-friendly and recyclable path to a more sustainable industry [1] .
The goal of this research is to add even more value to this wonderful natural resource by improving the processing procedures used to prepare silk. If it is possible to create a more eco-friendly and green process to remove sericin from silk fabric, then the value of the fiber itself will be increased.
Silk can be the next truly eco-friendly green fiber used in many kinds of textile products [2] [3] . The secondary goal would be the creation of new sericin based products for the cosmetic and medical industries. The status of silk as the source of the protein could potentially increase cosmetic product value [4] .
To reach these goals, research must be made to solve some of the conventional processing of silk fibers to eliminate or reduce some of the current practices. Conventional textile industry processes to remove silk sericin using acid, alkaline and high temperature water. This removal process includes using boiling water and sometime adding alkaline solutions such as: NaOH, Na2CO3, Na2SiO3, NaHCO3, Na4P2CO7, NH4OH. This traditional “degumming” process not only consumes a high amount of wasted energy and produces high temperatures but also adds a lot chemicals to the environment and consumes a lot of clean water [5] [6] .
Raw Silk fibers must have the sericin and others natural unwanted contaminants removed before use in textile production. Raw silk may contain such other contaminants as oil wax, natural pigment and dust. This process uses a lot of hot water to remove those contaminants [7] . The wasted clean water and thermal energy is extensive and damaging to the environment. Just one of the conventional ways to remove silk sericin is to add synthetic soap into a solution and boil the solution up to 95 degree C for 1 hour. The estimated output of this process is that every 100 kg of raw silk can produce up to 22 kg of sericin. The textile industry needs to develop additional, greener ways of “degumming”.
Sercin has a molecular weight between 10,000 and 300,000 Da [8] [9] . The normal silk cocoon contains 70% to 80% fibroin and sericin makes up most of the remaining 20% to 30% of the total cocoon weight. Therefore, the process of degumming may discharge a lot of waste water which contained sericin protein. The large amount of waste water used contains chemically treated sericin with a high chemical oxygen demand (COD) level. This catalyst led to some promising experiments in developing a sustainable process to remove sericin from raw silk fiber. Some of the initial findings show that the sericin byproduct remains clean and can be easily collected as a secondary product to be used in the cosmetic or medical industries. This new, inventive and green degumming process may create a significant recycling economic and social benefit to textiles and the agriculture Industries [5] .
CO2 super critical fluid has been used in processing foods and pharmaceuticals for removal of small substances since the early year1980 [10] . Over the years, several reports have been filed on the use of CO2 super critical fluid for many different uses. There have been studies on conventional silk fibers degummed with a process of adding citric acid [11] can remove almost 100% of sericin. The food industry uses it to extract plant substances, polymer engineers use it to engineer new polymer structures and the textile industry has recently started to use it in the dyeing process to save wastewater [10] [12] . Using CO2 super critical fluid is a mature, proven process for several applications. Our experiments are leading to a new use of this safe substance [13] .
Our research reference above reports that using CO2 super critical fluid to remove sericin from raw silk fiber. The remove sericin process can be used to large scale textile industry [12] . The environmental benefit from textile industry using this advance remove sericin process can benefit from less water and less energy consumption [3] .
2. Materials and Methods
2.1. Materials
Commercial grade silk fiber in the form of a plain woven textile. Decorticated silk fiber (Bombyx Mori) used in these study was collected from Taipei city, Taiwan. Silk fiber is cut into 5 cm × 8 cm with a weight between 2 g to 4 g for super CO2 critical degumming test.
2.2. Methods
Nine raw silk samples were made. Each sample was 5 cm × 8 cm in size. Six silk samples were pretreated in an open bath containing either citric acid or tartaric acid to remove the impurities.
These impurities could include oil, dust contamination and unwanted dye. The citric acid or tartaric acid [11] concentration PH 2 - 3, of 5 % w/w for 6 - 8 hours at room temperature. The remaining 3 raw silk samples were used as control for conventional NH4OH treatment.
The experimental process starts by preparing a solution for raw silk degumming. The liquid to silk ratio is in the range of 1:8 to 1:12. The liquid may consist of pure water or deionized water.
Organic acids are prepared. Acids used may include: acetic acid, citric acid, tartaric acid. Mixing the organic acids in purified water to modulate the acidity to the range of PH2 ~ 3. The raw silk was added to the solution and allowed to soak for 6 - 8 hours.
The raw silk is removed from the bath and moved to the supercritical carbon dioxide containers.
The proper surfactant is added at this time. Surfactants may include: glycol type nonionic surfactant, lipidation glycol type nonionic surfactant, lauryl alcohol, sodium lauryl sulfate.
The supercritical carbon dioxide containers are heated to temperatures between 95 to 127 degree C. CO2 levels are kept between 150 - 400 atm, for a processing time between 45 to 70 minutes.
Carbon dioxide emissions are removed and recycled for later use. This process is referred to as SC CO2 degumming for cleanly removing sericin from raw silk. Directly after the processing time the raw silk is cleaned of the sericin with the appropriate.
2.3. Testing
The fineness was tested according to GB/T12411.3. The constituent contents were tested according to GB/5889?86, and gum decomposition was calculated using Equation (1):
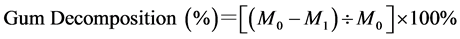 (1)
(1)
where M0 is the gum content of the raw silk fiber, M1 is the residual gum content of the degummed silk fiber.
2.4. Results and Discussion
Fibroin is an insoluble protein that comes from silk (Mombx Mori.) Fibroin is one of the two silk proteins that raw silk consists of. Fibroin protein is the protein at the center of a raw silk strand. Sercin is the second protein that creates a sticky protein coating around the fibroin to function as a glue to hold together a silk worm cocoon. To remove the sericin protein from the fibroin core of the raw silk strand, we use either a citric or tartaric acid pretreatment. The pretreatment solution is intentionally kept between a pH of 2 - 3. This is below the pH levels of both fibroin (3.6) and sercin (3.3). Due to the isoelectric point of the silk proteins being higher than the pretreatment fluid the isoelectric polarization of proteins switches to a positive charge [14] .
The citric acid or tartaric acid contains a carboxylic acid group which we use to dissociate the silk proteins by replacing the hydrogen bonds with carboxylic acid groups. The silk protein will carry a positively charged hydrogen ion (proton). Replacing hydrogen bonds with carboxylic acids helps to destabilize the amino acid structure of the silk protein. The addition of a surfactant creates a hydrophilic site under super critical carbon dioxide conditions. Once processed, a water bath can remove the sercin from the fibroin reactively easily.
It can be seen from the following three SEM figures that degumming silk fibers by the super critical CO2 fluid have the best surface. Figure 1 shows the untreated silk fibers still bonded together with the sticky sericin protein. Figure 2 shows the silk fibers after the conventional degumming process and Figure 3 shows the unbounded silk fibers after the removal of the sericin using the super critical CO2 process.
Figure 1. Commercial grade before degumming.
Figure 2. Commercial grade silk fiber after degumming with 3% Na2CO3 solution.
Figure 3. Commercial grade silk fiber after degumming with supercritical CO2 process.
3. Color Dyeing Ability Testing
In this study we dyed 3 types of treated silk fabrics. Three types of silk samples were prepared and weighted. (1) Without scouring treatment, (2) Conventional scouring treatment and (3) Supercritical carbon dioxide process.
The dye was provided by Tainaset dye Company. They produce 3 types of deep shade acid dyes: Red 2G-T, Yellow 2RN-T, and Navy Blue RN-T. The silk fabric was tested by using Red, Yellow and Navy blue deep shade acid dyes. The silks were all tested at same shade percentage of 1% concentration. First, the scouring treatment was carried out. Then the dyeing liquid along with the silk samples were heated to 90 degrees Celsius. After dyeing liquid reaches 90 degree C it is kept at that temperature for 30 to 40 minutes. Reflectance (%) of the dyed fabric samples were measured after dyeing. The color strength of a dyed fabric is usually expressed by its color strength (K/S).
Dyeing curve of silk fabrics.
Results and Discussion
The sample group of untreated silk had the highest color strength in all 3 colors. The sample group of silks treated with the SC CO2 process had the second highest color strength in all 3 colors. The sample group silk treated with the conventional treatment process had lowest color strength number.
The tests show little difference in the dying process of silk fibers when comparing the sample group using the supercritical carbon dioxide process to the conventional scouring process. This stays consistent with all three colors of dyes tested.
Untreated silk fibers contain two basic proteins, fibroin and sericin. In the dying process both of these proteins retain dye. This resulted the untreated silk samples ending with color strength K/S number almost two times higher than treated silk fibers. This is also consistent with conventionally treated silk fibers. The removal of sericin using either method makes it difficult for the silk fibers to retain dye but both processes are relatively equal to each other as a degumming process.
4. Conclusions
The above testing shows raw silk pretreatment by using organic acids in the range PH2 - 3 which includes: acetic acid, citric acid, tartaric acid in addition to adding a surfactant such as: glycol type nonionic surfactant, lipidation glycol type nonionic surfactant, lauryl alcohol, sodium lauryl sulfate is a very efficient new way to remove sericin from raw silk when using a supercritical carbon dioxide process.
The main benefit of using this method is less waste water, less energy consumption and the use of bio-renewable materials for maintaining a global CO2 balance at stable level [1] . There are additional commercial benefits in producing a clean sericin, including its use in the medical and cosmetic industries. These tests accomplish this secondary goal.
These tests show amazing promise in revolutionizing the very large, yet very unsustainable industry of silk processing. Using renewable biopolymers rather than the more environmentally destructive conventional pretreatments is a beneficial step in the right direction. Using a finishing process that takes advantage of CO2 supercritical fluid rather than needing to produce huge amounts of thermal energy and toxic waste water makes this new procedure critical to pursue for environmental reasons.
Acknowledgements
The authors are grateful to Oriental university material and textile department Professor Lin, Shang-Ming and graduated student Jane, Wen-Yang for necessary helping in CO2 supercritical fluid equipment of these studies.
Cite this paper
Lo, C.-H. and Chao, Y.C. (2017) Degumming of Silk Fibers by CO2 Supercritical Fluid. Journal of Materials Science and Chemical Engineering, 5, 1-8. https://doi.org/10.4236/msce.2017.54001
References
- 1. Drzal, L.T., Mohanty, A.K. and Misra, M. (2001) Bio-Composite Materials as Alternatives to Petroleum-Based Composites for Automotive Alications: An Overview.
- 2. Wang, W., Cai, Z. and Yu, J. (2008) Study on the Chemical Modification Process of Jute Fiber. Journal of Engineered Fibers and Fabrics, 3.
- 3. Edward, R., Sun, Q., Zhang, Z., Zhang, C. and Gou, W. (2009) Mini-Review: Green Sustainable Processes Using Supercritical Fluid Carbon Dioxide. Journal of Environmental Sciences, 21, 720-726.
- 4. Vepari, C. and Kaplan, D.L. (2007) Silk as a Biomaterial. Progress in Polymer Science, 32, 991-1007.
- 5. Wu, J., Wang, Z. and Xu, S. (2008) Enzymatic Production of Bioactive Peptides from Sericin Recovered from Silk Industry Wastewater. Process Biochemistry, 43, 480-487.
- 6. Sargunamani, D. and Selvakumar, N. (2006) A Study on the Effects of Ozone Treatment on the Properties of Raw and Degummed Mulberry Silk Fabrics. Polymer Degradation and Stability, 91, 2644-2653.
- 7. Capar, G., Aygun, S.S. and Gecit, M.R. (2009) Separation of Sericin from Fatty Acids towards Its Recovery from Silk Degumming Wastewaters. Journal of Membrane Science, 342, 179-189.
- 8. Vaithanomsat, P. and Kitpreechavanich, V. (2008) Sericin Separation from Silk Degumming Wastewater. Separation and Purification Technology, 59, 129-133.
- 9. Fabiani, C., Pizzichini, M., Spadoni, M. and Zeddita, G. (1996) Treatment of Waste water from Silk Degumming Processes for Protein Recovery and Water Reuse. Desalination, 105, 1-9.
- 10. Kikic, I. (2003) Supercritical Impregnation of Polymers. Current Opinion in Solid State and Materials Science, 7, 399-405.
- 11. Rahman Khan, M.M., Tsukada, M., Gotoh, Y., Morikawa, H., Freddi, G. and Shiozaki, H. (2010) Physical Properties and Dyeability of Silk Fibers Degummed with Citric Acid. Bioresource Technology, 101, 8439-8445.
- 12. Gerardo, M.A., Smith, A., Carl, B., Walter, H.A. and Donald, B.L. (2000) Supercritical Fluid Technology in Textile Processing: An Overview. Industrial & Engineering Chemistry Research, 39, 4806-4812.
https://doi.org/10.1021/ie0002475 - 13. Perrut, M. (2000) Supercritical Fluid Alications: Industrial Developments and Economic Issues. Industrial & Engineering Chemistry Research, 39, 4531-4535.
https://doi.org/10.1021/ie000211c - 14. Zhang, G. and Lu, C. (2009) Studies on Charges of Silk Fibroin. Silk Science, 35, 99-105.





