Open Journal of Ecology
Vol.08 No.04(2018), Article ID:83840,11 pages
10.4236/oje.2018.84015
Rhizogenesis of Two Species Fabaceae: Cicer arietinum L. and Pisum sativum L.
Beddi Mohammed, Benabadji Noury
Faculty of Nature and Life Sciences, Tlemcen University, Tlemcen, Algeria

Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 6, 2018; Accepted: April 16, 2018; Published: April 19, 2018
ABSTRACT
Our work of comparative study on the biomorphological, rhizogenic level of Chickpea (Cicer arietinum) and Pea (Pisum sativum), includes successively the following results: The in-vitro culture done, showed strains, which can reach 6.5 mm in the medium Nutrient agar (NA) for Cicer arietinum 6 mm of strain were recorded in the PDA culture medium for Pisum sativum. The best strain is obtained in temperature of 20˚C (ambient). According to the results of the rhizogenesis, we notice that the nutrient agar (NA) medium responds better that the PDA medium for Cicer arietinum. Meanwhile, the medium PDA brings a better reaction (response) compared with the nutrient agar medium concerning Pisum sativum.
Keywords:
Pisum sativum L. (pea), Cicer arietinum L. (chickpea), Rhizogenesis

1. Introduction
The rhizogenesis is a complicated phenomenon, which contains various phases: the dedifferentiation, the formation of heap of meristematic cells, differentiation and organization of the meristematic heap into root primordium, which will develop in young roots [1] [2] .
It implies the study, understanding and the comparison of the Rhizogenic as well (roots growth) of Pisum sativum and Cicer arietinum, of various origins, final percentages of rhizogenesis.
The in vitro culture (also called propagation) is a technique aiming the regeneration of a whole plant from cells or from plant tissues in nourishing medium, by using techniques of cell cultures [1] [3] [4] [5] .
The in vitro culture techniques are rather close to surgical and micro-surgical ones, they require like them, a lot of care in the preservation of the conditions of asepsis because the presence of a single bacterium or fungus, is enough to invade a culture medium. That’s why it is better to respect the maximum of rules, even if it means simplifying them. It would thus be necessary to watch that all the sterilized instruments are put down in the sterile sphere, so as to have the slightest gesture to be made during the manipulations and in the sterile air [6] [7] [8] [9] .
Very widespread in our region, Cicer arietinum and Pisum sativum are a biological material with interesting reproductive capacities (rapid meristem development). A little work has been done on the rhizogenesis of these two species (Cicer arietinum and Pisum sativum).
2. Materials and Methods
2.1. The Following Equipment Was Used on the Laboratory
・ Flasks of Nutrient agar (NA);
・ Potato Dextrose Agar’s flasks (PDA);
・ Ethylic alcohol in 95%;
・ Bleach;
・ Distilled water;
・ Lactic acid 1 ml: to stop the bacterial growth;
・ Antifungal Nystatine 50 mg/l, to prevent the growth of (fungi) mushrooms;
・ Rootlets of Cicer arietinum and Pisum sativum.
2.2. Composition of Cultural Media (PDA, NA) (Table 1)
Table 1. Chemical compositions of cultural media.

2.3. Methodology
We followed the classical methods used in plant physiology laboratory in general and those morphogenesis in particular.
The preparation of culture media is made the following protocol:
Every medium of culture prepared from a dehydrated medium, (23 g/l) of Nutrient agar (NA) and (39 g/l) of PDA, incorporated into a liter of distilled water; the whole being warmed until boiling. The medium is then distributed in autoclaved flasks during 20 minutes in 120˚C.
The rhizogenic culture, we follow the methodology below:
Cultural media (Nutrient agar (NA) and PDA) were liquefied in Mary’s bath for 30 minutes. We add in the medium by surfusion (45˚C) a dose of a milliliter of lactic acid and 50 mg/l of Nystatine. Then, we pour the media into Petri dishes between two beak-bunsen, boxes are maintained opened to dry them in front of the flame to avoid the formation of water droplets on the lid. The lock of Petri dishes is necessary to leave the media (Nutrient agar (NA) and PDA) solidifying and also to avoid any risk of contamination.
The roots’ extremities were dipped into the bleach, and then cut by means of a blade sterilized in 2 mm fragments, because the growth is strictly sub-terminal. Indeed, it’s at the level of the apex that sites responsible for the rhizogenesis are needed, and which are searched by [10] , then disinfected in an alcoholic bath in 95˚C for 30 seconds. After that, we rinsed them in three baths of sterilized distilled water.
The fragments of roots are quickly dried in a sterilized paper-filter, then stuck again in culture media by means of a sterilized crowbar.
The boxes’ lock is essential for the prevention. The preservation of boxes is made in the weather conditions of the laboratory, the temperature varies between 5˚C, 20˚C and 40˚C). All this to assure the “in vitro” vegetative multiplication which corresponds to the rhizogenesis phenomena. For the study of this phenomenon of implanting, an illumination of supplement favors the cuttings’ implanting of certain species.
3. Results and Interpretations of Rhizogenic Culture
3.1. Strain of the Roots of Pisum sativum at Room Temperature of 20˚C, (Figure 1, Table 2, Photo 1(a) & Photo 1(b)) Photo
After three weeks of culture, we obtained rootlets, from the treated roots. The development of the roots of Pisum sativum showed a linear evolution at an ambient temperature of 20˚C in culture media. The PDA medium records a growth which went beyond 3.5 mm (first week), 5 mm then 6 mm (the third week). Concerning the nourishing agar medium, it shows a linear growth where
Figure 1. The rootlets strain histogram of Pisum sativum in two culture media (NA and PDA) at room temperature of (20˚C).
Table 2. Elongation of rootlets (Pisum sativum) at room temperature (20˚C).
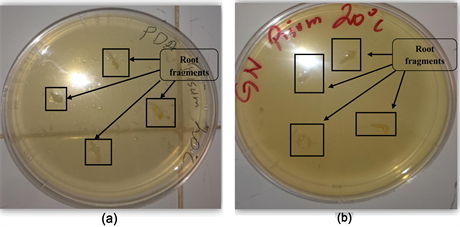
Photo 1. (a) Rhizogenic phase in the PDA medium at room temperature 20˚C, (b) Rhizogenic phase in the NA medium at room temperature 20˚C.
thresholds are slightly lower than those of the PDA medium. We go beyond 3 mm (first week), 4 mm (the second week) and 5.5 mm (the third week).
3.2. Roots Strain of Pisum sativum at Cold Temperature (5˚C), (Figure 2, Table 3, Photo 2(a) et Photo 2(b))
During these three weeks of culture, we obtained small rootlets, from the beforehand treated roots. The PDA medium seems to show rather a significant growth where the size oscillates between 1.5 mm (first week) and 4 mm (the third week). Concerning the Nutrient agar (NA) medium, this one gives us a relatively lower growth comparing to the PDA medium. It varies between 1.5 mm (first week) and 2.5 mm (the second week) to increase in the third week and reach 3 mm.
3.3. Roots Strain of Pisum sativum at Temperature (40˚C), (Table 4, Photo 3(a) and Photo 3(b))
At 40˚C, the roots development of Pisum sativum does not simply take place in both media (Nutrient agar (NA) and PDA) and this happens of course during the three weeks. The reasons in our opinion are:
・ The environment of culture loses its wanted consistency in contact with the temperature in question (40˚C in the steam room), and by this fact prevents the experiment’s progress (root strain);
Figure 2. Rootlets strain histogram of Pisum sativum in two culture media (NA and PDA) at cold temperature (5˚C).
Table 3. Elongation of rootlets (Pisum sativum) at cold temperature (5˚C).
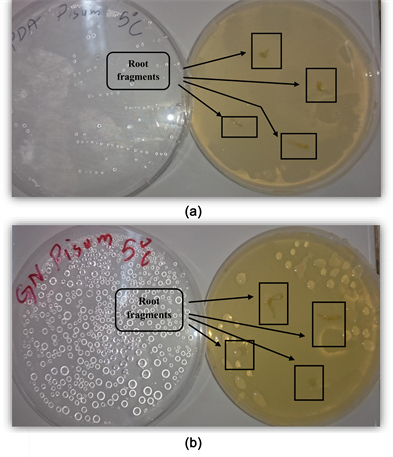
Photo 2. (a) Rhizogenic phase in the PDA medium at a cold temperature 5˚C, (b) Rhizogenic phase in the NA medium at a cold temperature 5˚C.
Table 4. Elongation of rootlets (Pisum sativum) at temperature 40˚C.
・ On the other hand, the pathogenic agents multiplication which inhibits considerably this strain;
・ It is possible also that our media in spite of the multiple precautions did not keep their infertility (essential for this kind of work).
3.4. Roots Strain of Cicer arietinum at Room Temperature (20˚C), (Figure 3, Table 5, Photo 4(a) and Photo 4(b))
After three weeks of culture, we obtained rootlets, from the handled roots, the development of the roots of Cicer arietinum showed a linear evolution at an ambient temperature of 20˚C in cultural media. The nutrient agar (NA) medium records a growth which passed 3 mm (first week), 4.5 mm then 6.5 mm (the third week). Concerning the medium PDA, it shows a linear growth where
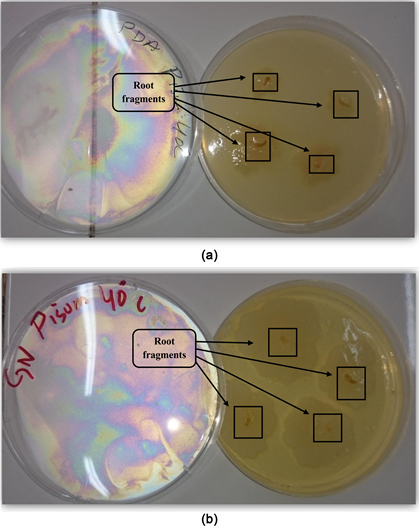
Photo 3. (a) Rhizogenic phase in the PDA medium at a temperature 40˚C, (b) Rhizogenic phase in the NA medium at a temperature 40˚C.
Figure 3. Rootlets strain histogram of Cicer arietinum in two cultural circles (NA and PDA) at a room temperature of 20˚C.
Table 5. Elongation of rootlets (Cicer arietinum) at room temperature (20˚C).
Photo 4. (a) Rhizogenic phase in the PDA medium at a room temperature 20˚C, (b) Rhizogenic phase in the NA medium at a room temperature 20˚C.
thresholds are slightly lower than those of nutrient agar (NA) medium. We pass 2.5 mm (first week), 4.5 mm (the second week) and 6 mm in the third week.
3.5. Roots Strain of Cicer arietinum in Cold Temperature (5˚C), (Figure 4, Table 6, Photo 5(a) and Photo 5(b))
During these three weeks of culture, we obtained small rootlets at least, from the beforehand handled roots. The nutrient agar (NA) medium seems rather to show a significant growth where the size oscillates between 2 mm (first week) and 4.5 mm (the third week). Concerning the PDA medium, this one gives us a relatively lower growth compared with the nutrient agar medium. It varies from 1.5 mm (first week) to 2 mm (the second week), to increase in the third week and reach 3 mm.
3.6. Roots Strain of Cicer arietinum at a Temperature of 40˚C, (Table 7, Photo 6(a) and Photo 6(b))
At 40˚C, the development of the roots of Cicer arietinum does not simply take place in both media (nutrient agar and PDA), and this of course occurs during three weeks, There is always a percentage of infection which can be caused by the contamination during our experience.
4. Conclusions
We made an in vitro experiment on two family-species of fabacées (Cicer arietinum and Pisum sativum), it concerned particularly the experiences which took
Figure 4. Rootlets strain histogram of Cicer arietinum in two cultural circles (NA and PDA) at a cold temperature of 5˚C.
Table 6. Elongation of rootlets (Cicer arietinum) at cold temperature (5˚C).
Photo 5. (a) Rhizogenic phase in the PDA medium at a cold temperature 5˚C, (b) Rhizogenic phase in the NA medium at a cold temperature 5˚C.
Table 7. Elongation of rootlets (Cicer arietinum) at temperature 40˚C.
Photo 6. (a) Rhizogenic phase in the PDA medium at a temperature 40˚C, (b) Rhizogenic phase in the NA medium at a temperature 40˚C.
place in the laboratory on two cultural media of different chemical compositions (nutrient agar and the PDA medium). The work concerned the root activities (rhizogenèse) of these plants in different temperatures, and we compared the results obtained between these two species as well.
The in vitro culture was made to follow the strain of rootlets and their development (rhizogenesis), the results at the end of three weeks in (20˚C and 5˚C) showed that the implanting of Cicer arietinum is relatively better than the one of Pisum sativum.
We also notice a total absence of the root strain at 40˚C.
According to the results of the rhizogenesis, we notice that the nutrient agar (NA) medium responds better that the PDA medium for Cicer arietinum. Meanwhile, the medium PDA brings a better reaction (response) compared with the nutrient agar medium concerning Pisum sativum.
We deduced among others a contamination by the pathogenic agents at the level of these media, from different origins (bacterial or fungal). The proliferation of the infections appeared at the level of the contact zone between roots and these media. We think that it is the explants which are the source of infection because roots were submitted to an incomplete sterilization.
In this study, we suggest that this work on the root strain should be pursued, by trying to increase or to vary the temperatures which are between those studied (treated) cells, to try to find the variables obtaining (desired biological responses).
Cite this paper
Mohammed, B. and Noury, B. (2018) Rhizogenesis of Two Species Fabaceae: Cicer arietinum L. and Pisum sativum L. Open Journal of Ecology, 8, 239-249. https://doi.org/10.4236/oje.2018.84015
References
- 1. Margara, F. (1989) Bases de la multiplication végétative: Les méristèmes et l’organogénèse. Ed INRA, Paris, 262 p.
- 2. Chatibi, A., Kchouk, M.L., Benabdellah, F., Zemmi, H. and et Ghoibl, A. (1995) Rooting Improvemnet of Pistacia vera L.C.V Mateur by in Vitro Culture of Apices and Cuttings. Acta Horticulture, 419, 213-220. https://doi.org/10.17660/ActaHortic.1995.419.34
- 3. Margara, F. (1982) La multiplication végétative in-Vitro. Aspects généraux. Agro., 15, 701-711.
- 4. Boxus, P. (1995) Multiplication végétative: Micro-propagation et Embryogénèse somatique in biotechnologies végétales. BV 93, Ed Cnep. Aupelf-Uref. 191 p.
- 5. Semal (1998) Reproduire à l’identique: Mythe et réalité. Cahier Agriculture, No. 7, 6-8.
- 6. Skirvin, R., Cogner, M., Norton, A., Motoika, S. and et Gorvin, D. (2000) Somaclonal Variation: Do We Know What Causes It. Agbiotech Net., 12, 48.
- 7. Morel, M. (1956) Nouvelles méthodes permettant de réaliser des cultures de tissus végétaux. Rev. Gén. Bot., 63, 314-325.
- 8. Boulay, J. (1993) La culture in Vitro et ses applications à des plantes carnivores. Club Drosera de Metz. Univ. Nancy, 1.
- 9. Monnier, M. (1971) Action des conditions de stérilisations sur la valeur nutritive des milieux utilisés pour la culture des embryons isolés de Capsella bursapastoris. Rev. Gén. Bot., 78, 57-60.
- 10. Augé, R., Beauchesne, G., Boccon-Gibod, J., Decourtye, L., Digat, B., Jalouzot, R., Minier, R., Morand, J., Reynoird, J. and et Strullu, D. (1989) La culture in Vitro et ses applications horticoles. 3éme édition revue, corrigée augmentée. Ed. Tech. Doc. Lavoisier 225 p.







