Synthetic of Some New Fluorine Compounds Bearing 1,2,4-Triazine Moieties and the Related Hetero-Polycyclic Nitrogen Systems as Pharmacological Probes-Overview ()
Keywords:

1. Introduction
Recently, fluorine substituted 1,2,4-triazine derivatives have been gathering considerable interest in various applications in pharmaceuticals activities and chemotherapy fields due to have a wide range of medicinal treatment as anti-HIV [1] , anti-fungal [2] , anti-cancer [3] , anti-inflammatory [4] , as cyclin-dependent kinases (CDK) [5] [6] , anti-microbial activities [7] , and antioxidant agents [8] . Most of the studies addressing synthesis and chemistry of fluorinated hetero-cyclic have been related to drug discovery research [9] [10] . It is interesting that replacing hydrogen and other functional groups with fluorine atoms can have a dramatic effect on the modulation of electronic, lipophilic, and steric parameters, all of which can critically influence both the pharmacodynamic and pharmacokinetic properties of drugs. Based upon these results, the present overview reports an important route of fluorine compounds substituted 1,2,4-triazine with the study of chemical reactivities and evaluation of the effects on the vital biological process.
2. Synthesis of 3-Amino/Mercapto-5,6-Difluoro-Substituted-1,2,4-Triazine
2.1. Synthesis
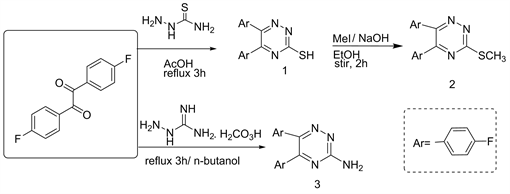
Musator et al. [11] synthesized 3-mercapto/methyl Thia-5,6-di(4'-fluoro-phenyl)-1,2,4-triazine (1 and 2) from refluxing 4,4'-difluorobenzine with thiosemicarbazide in glacial acetic acid followed by methylation via treated with MeI/NaOH/EtOH to yield 2 (Scheme 1). Similarly, refluxing 4,4'-difluorobenzil with aminoguanidine bicarbonate in n-butanol yielded 3-amino-5,6-di (4'-fluorophenyl-1,2,4-triazine (3) (Scheme 1) [6] .
2.2. Reactivity
3-Amino-5,6-difluorophenyl-1,2,4-triazines are important intermediates in the synthesis of isolated and fused heterobicyclic nitrogen systems as biological agents. Thus, Makki et al. [6] synthesized some fluorinated 1,2,4-triazine bearing other heterocyclic moieties as cyclin dependent kinases (CDK) (Scheme 2). Thus, 3-amino-6,7-di (4'-fluorophenyl)-imidazo [3,2-b] [1,2,4]triazine (4) and 6,7-di(4'-fluorophenyl)-2,3-dihydro-3-oxo-imidazo [3,2-b] [1,2,4-]triazine (5) obtained from refluxing compound 3 with chloroacetonitrile and monochloroacetic acid in DMF respectively (Scheme 2) [6] .
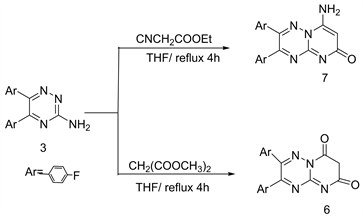
Cyclization reactions of 3-amino-1,2,4-triazine 3 with dimethyl malonate and ethyl cyanoacetate in refluxing THF produced pyrimido [3,2-b] [1,2,4]triazin-2,4-dione (6) and 4-amino-pyrimido-[3,2-b] [1,2,4]triazine-2-one (7) respectively (Scheme 3) [6] .
The possible mechanism for the formation of compound 7 is in shown in Figure 1. Also, the structure of 7 deduced from mass fragmentation pattern is reported Figure 2.
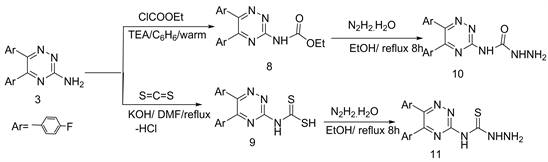
Semicarbazide and thiosemicarbazide derivatives used as starting material for the building of new hetero-polycyclic nitrogen systems as pharmacological probes [12] [13] . Thus, acylation of 3-amino-triazine 3 via treatment with ethylchloroformate in dry benzene and CS2/KOH followed by hydrazinolysis (heated at reflux with hydrazine hydrate in EtOH) produced N4-(1,2,4-triazin-3'-yl) semicarbazide/thiosemicarbazide derivatives 10 and 11, respectively (Scheme 4) [6] .
Scheme 1. Formation of compounds 1-3.

Scheme 2. Formation of compounds 4 and 5 from 3.
Scheme 3. Formation of compounds 6 and 7 from 3.
Scheme 4. Formation of compounds 8-11 from 3.
![]()
Figure 2. Mass fragmentation pattern of compound 7.
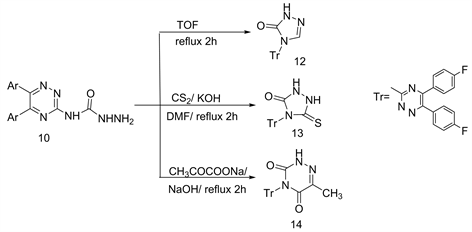
Under the experimental conditions, the ring closure reaction of compound 10 by refluxing with triethyl orthoformate, CS2 (DMF) and sodium pyruvate (aq.NaOH) yielded the 1,2,4-triazolone 12 and1,2,4-triazolthion 13 and the 6-azauracile 14, respectively (Scheme 5) [6] .
The structure of compound 13 was deduced using the mass fragmentation pattern (Figure 3) [6] .
Similarly, hetero-cyclization of compound 11 under the last same conditions and reagents lead to the direct formation of 1,2,4-triazol-3-thions 15 and 16 and/or 3-thioxo-4-[5,6-di(4'-fluoro-phenyl)1,2,4-triazin-3'-yl]-6-methyl-1,2,4-triazin-5-one (17), respectively (Scheme 6) [6] .
On other hand, fully fluorinated thiobarbituric acids bearing 1,2,4-triazine moieties 19 obtained from the interaction between compound 3 with 9 in refluxing EtOH to give N, N'-disubstituted thiourea 18, which upon ring closure reactions with malonic acid in refluxing glacial acetic acid yielded the target 19 (Scheme 7) [8] .
![]()
Figure 3. Mass fragmentation pattern of compound 13.
Scheme 5. Formation of compounds 12-14 from 10.
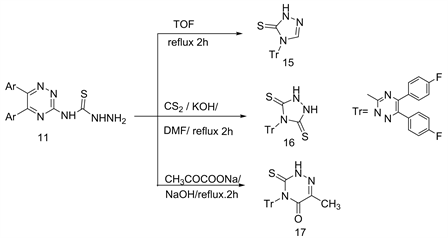
Scheme 6. Formation of compounds 15-17 from 11.
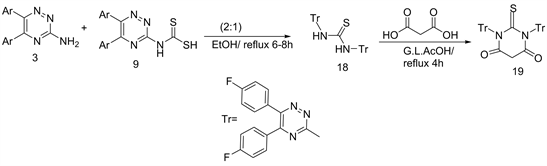
Scheme 7. Formation of compounds 18 and 19.
The CDK2 inhibitory activity of the compounds 3-9 evaluated in comparison with olomoucine as standard according the reported method [14] , where the highly inhibitor effects increase in the order 11 > 13 > 16 > 17 > 3. The compound 16 exhibit a good effect toward the tumor cells damage as the olomoucine. Also, the in vitro antitumor testing of the highly active compounds evaluated according the reported method [15] under different concentration. A sulforhodamine B (SRB) protein assay was used to estimate cell viability or growth by determining GI50, TGI, and LIC50. Compound 11 showed the anticancer activity against non-small cell lung, renal, and breast cancer cell, while compound 13 exhibit anti-cancer of type leukemia and breast cancer cell, compound 16 showed anti-cancer activity against non-small cell lung cancer, finally, compound 17 exhibit anti-cancer of type breast cancer [6] .
3. Synthesis of Fluorine Compounds Substituted Fused Hetero-Bicyclic Nitrogen Systems Containing 1,2,4-Triazines
3.1. Synthesis
Due to a highly resistance of microorganisms towards the anti-biotic uses, Aqlan et al. [16] synthesized new fluorine substituted pyrimido-1,2,4-triazinones as plant protection of wheat grain from fungi infection by using 2-hydrazino-4-(4'-fluorophenyl)-6-oxo-pyrimidine-5-carbonitrile (20) as a nucleophilic reagents attack of various functional reagents as electrophilic to produce the new fluorine compounds.
Cyclocondensation of 2-hydrazino-pyrimidinoe 20 with 1,2-bicarbonyl compounds such as sodium pyruvate/aq. NaOH or diethyl oxalate (THF) under refluxing 2h produced 8-(4'-fluorophenyl)-7-cyano-3-methyl-pyrimido [3,2-c] [1,2,4]triazin-4,6-dione (21) and 8-(4'-fluorophenyl) -7-cyano-1,2,3,4-tetra hydro pyrimido-[3,2-c] [1,2,4]triazin-3,4,6-trione (22), respectively (Scheme 8) [16] . The interaction between compound 20 with (E)4-aryl-2-oxo-but-3-eneoic acid in refluxing aq.NaOH, yielded 8-(4'-fluorophenyl)-7-cyano-3-styryl-1H-pyrimido [3,2-c] [1,2,4]triazin-4,6-diones (23) and not 24 (Scheme 9) [16] .
Formal structure of compound 23 deduced from spectral measurements. Mass spectrometric study were recorded a molecular ion peak that the base peak Figure 4 [16] .
Due to the important properties of fluorinated heterocyclic substituted indole moieties for their applications [17] [18] . Thus, interaction between 2-hydrazino-pyrimidinone 20 and isatin in refluxing aq.NaOH or DMF yielded 8-(4'-fluoro-phenyl)-7-cyano-3-(2'-aminophenyl)-1H-pyrimido [3,2-c] [1,2,4]triazine-4,6-dione (25) or 11-(4'-fluorophenyl)-10-cyano-1H-pyrimido [3,2-c] [1,2,4]triazino-[6,5-b]indole(26), respectively (Scheme 10) [16] .
A regioselective hetero-cyclization of 2-hydrazino-pyrimidinone 20 towards α-active electrophilic agents [19] [20] as monochloroacetic acid (aq.NaOH) and chloroacetyl chloride (DMF) under warming leads to the direct formation of fluorinate pyrimido-triazinones 27 and/or 28, respectively (Scheme 11). Both the compound 27 and 28 are considered an isomeric structure [16] .
3.2. Reactivity
The mass fragmentation pattern of compounds 21 and 26 give us a good indication about their stability Figure 5 and Figure 6 [16] , were the base peaks in these compounds are 95 (4-fluorophenyl) ions.

Scheme 8. Formation of compounds 12 and 22 from 20.
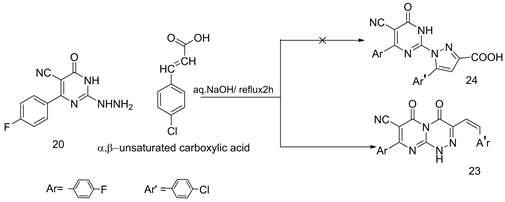
Scheme 9. Formation of compound 23.
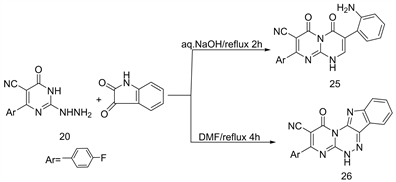
Scheme 10. Formation of compounds 25 and 26 from 20.
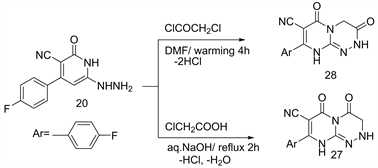
Scheme 11. Formation of compounds 27, 28 from 20.
![]()
Figure 4. Mass fragmentation pattern of 23.
![]()
Figure 5. Mass fragmentation pattern of 21.
![]()
Figure 6. Mass fragmentation pattern of 26.
The presence of a free amino group in the structure of 25 was established from Fluoroacylation by warming with hexafluoro-acetic anhydride (DMF) or fluoro-aroylation by warming with 4-fluoro-benzoyl chloride (DMF), afforded the N-(trifluoroacetamido) 29 or N-(4'-fluoro benzamido) derivatives 30, respectively (Scheme 12) [16] .
Oxidation of 27 and 28 via refluxing with Fe2(SO4/CH3OH yielded the tautomeric structures 31 and 32, respectively (Scheme 13) [16] .
The obtained compounds 21-32 evaluated both in vitro and in vivo of antifungal activity by inhibition of fungal mycelial growth of Alternaria alterata, helimen thosporium sativum and Fusarium moniliform according the reported methods [21] [22] [23] , where the compounds 23, 29 and 30 exhibit a high fungal toxicity activity. Prevention of blue mold development indicate the action of these compounds on the decay control on rind discs, were only the compounds 21 and 23 gave a good control at concentration at 500 mg/cm−1 against Alternaria alterata. The best germination (80% - 90%) was achieved by treating the seeds with a solution containing 1000 mg/ml of the compound 23 followed by 29 under the same concentration (59% - 70% germination) [16] . Similarly, the design, synthesis and molluscicidal activity of new phosphorus compounds bearing fluorine substituted 1,2,4-triazolo [5,1-c] [1,2,4]triazine derivative reported by Abdel-Rahman et al. [24] .
3.3. Synthesis
Refluxing 3-hydrazino-4-(4'-fluoro phenyl)-5-(pyridine-4'-yl)-1,2,4-triazole (33) with isatin in aq.NaOH or with diethyl oxalate in THF produced 3-(2-amino-phenyl)-8-(4'-fluorophenyl)-7-(pyridine-4'-yl)-1,2,4-triazol [5,1-c] [1,2,4]triazin-4(8H)-one (34) or 8-(4'-fluorophenyl)-7-(pyridine-4'-yl)-2,4-dihyro [1,2,4]-triazol [5,1-c] [1,2,4]triazin-3,4-dione(35), respectively (Scheme 14) [24] .
![]()
Scheme 12. Formation of compounds 29 and 30 from 25.
![]()
Scheme 13. Oxidation of compounds 27 and 28.
![]()
Scheme 14. Formation of compounds 34 and 35 from 33.
3.4. Reactivity
Phosphorylation of both compounds 34 and 35 by warming with chloro-diphenyl phosphate in the presence the DMF afforded the N-(diphenyl phosphiteamino) 36 and 37, respectively (Scheme 15) [24] .
The stability of compound 37 is indicated by the mass fragmentation pattern in Figure 7.
Compound 36 and 37 can be used as molluscicidal agents against the snails which cause the disease of Bilharziasis according via the reported method [24] , where the compound 37 exhibit higher activity than 36 in comparison with Bayluscids as a standard control (Table 1) [24] .
4. Synthesis of Fluorine Substituted Pyrazolo [4,3-e] [1,2,4]Triazines as Purine Analogues as (Condensed Systems)
4.1. Synthesis
Fluoroacylation of 4-aminoantipyrine (38) by warming with trifluoroacetic acid in THF yielded the N-trifluoroacetyl derivative 39, which upon heterocyclization by refluxing with hydrazine hydrate in abs.EtOH produced 2,3-dimethyl-1-phenyl-4H-5-trifluoromethyl-pyrazolo [4,3-e] [1,2,4] triazine (40), aroylation of 38 by warming with 4-fluorobenzoyl chloride in DMF produce the N-aryl amino 41. Ring closure reaction of 41 with aryl hydrazine in refluxing DMF gave 2,3-dimethyl-5-(4'-fluorophenyl)-6-(4'chlorophenyl)1-phenyl-pyrazolo [4,3-e] [1,2,4] triazine (42) (Scheme 16) [25] . Similarly, cyclocondensation of 38 with acid hydrazide in refluxing DMF yielded the pyrazolo-triazine 43, which on fluoroacylation produced N-trifluoroacetyl 44 (Scheme 16). The formation of 42 from 38 is shown in (Figure 8). Also, mass spectroscopy study of compound 44, were shown the molecular ion peak at low % with a base peak at m/z 198 (100%) attributes C12H12N3+ as Figure 9 [25] .
![]()
Table 1. The mortality of sanils at different concentrations.
![]()
Scheme 15. Formation of compounds 36 and 37.
![]()
Scheme 16. Formation of compounds 39-44 from 38.
![]()
Figure 7. Mass fragmentation pattern of 37.
![]()
Figure 8. Formation of compound 42 from 38.
![]()
Figure 9. Mass fragmentation pattern of compound 44.
The ddition of aryl isothiocyanate to compound 38 in warming DMF, yielded the N,N'-disubstituted thiourene 45, which upon hydrazinolysis in refluxing ethanol, produced 2,3-dimethyl-1-phenyl-4H-5-aryl amino-pyrazolo [4,3-e] [1,2,4]triazine (46), while addition of CS2 in aq.KOH to 38, followed by hydrazinolysis gave N4(substituted)thiosemicarbazide 47. Self-condensation of 47 lead to the formation 2,3-dimethyl-1-phenyl-4,5,5,6-tetrahydro-5-thioxo-pyrazolo [4,3-e] [1,2,4] triazine (48) Figure 10. Compound 48 also obtained [25] directly from refluxing 38 with thiosemicarbazide in acetic acid (Scheme 17). Formation of compound 48 from 38 may be tack’s place via the addition reaction between an amino-group of 38 and highly positive Carbone atom of CS2 followed by hydrazinolysis 47 and finally cyclocondensation via carbonyl group as shown in Figure 10.
Also, 2,3-dimethyl-1-phenyl-4,5,5,6-5-tetrahydro-5-oxo-pyrazolo [4,3-c] [1,2,4]-triazine (50) was produced from treatment of compound 38 with ethylchloroformate in worming C6H6-TEA followed by hydrazinolysis in refluxing THF (Scheme 18) [25] .
It is interesting that, addition compound 38 as nucleophilic agents to π-acceptor electrophilic as cyanamide in refluxing EtOH-piperidine as catalysis yielded the guanidine derivative 52, which on hydrazinolysis in DMF afforded 5-amino-2,3-dimethyl-1-phenyl-4H-pyrazolo [4,3-e] [1,2,4]triazine (53). Compound 53, also isolated from addition of H2NCN into compound 38 to give the amino-nitrile 54, followed by hydrazinolysis (Scheme 19) [25] .
4.2. Reactivities
Fluoroacylation of compounds 43, 50 and 53 by warming with trifluoroacetic acid in THF lead to the isolation of N-trifluoroacetyl derivatives 44, 51, and 55, respectively (Scheme 20) [25] .
![]()
Scheme 17. Formation of compounds 45, 46 and 48 from 38.
![]()
Scheme 18. Formation of compounds 49 and 50.
![]()
Scheme 19. Formation of compounds 52-54.
![]()
Scheme 20. Formation of compounds 44, 51 and 55.
The enzymatic properties of the synthesized compounds 40-55 were evaluated against purine metabolic enzymes at concentrations of 30 - 500 μM [26] [27] . The hexafluoroacetyl derivatives 51 and 55 were the strongest inhibitors with IC50 of 30 - 40 μm followed by trifluoroacetyl 44. Non-fluorinated derivatives exhibit much moderate to lethal inhibitor activity towards E. coli PNP an enzyme [25] .
5. Synthesis and Reactivity of Fluorine Compounds Substituted Hetero-Polycyclic Nitrogen Systems Containing 1,2,4-Triazino-Indole Moiety (Condensed Skelton)
Joshi et al. [28] reported some fluorine compounds containing 3-dialkyl aminoethyl thio-5-morpholino-methyl-1,2,4-triazino [5,6-b]indoles as having anti-bacterial, antifungal, and anti-viral activities (Figure 11). Also, Abdel-Rahman et al. [18] synthesized new fluorine substituted 3-amino-1,2,4-triazino-[5,6-b]-indoles derived from sulfa-drugs and fluorinated reagents as photochemical probes agents for inhibition of vitiligo disease. On other hand, novel herbicidal 3-dimethylamino-4H-1,2,4-triazino [5,6-b]indoles obtained by Mizutani et al. [29] (Figure 11) [30] [31] [32] . Recently, fluorine substituted 3-amino-1,2,4-triazino-indoles and/or 3-amino-imidazol-1,2,4-triazino-indoles have been used as anti-inflammatory agents [30] [31] .
3-Amino-8-fluoro-5H-1,2,4-triazino [5,6-b] indole (56) [30] (Scheme 21), was used to obtain a various of new fluorine compounds 57-61 via the treatment of 56 with different electrophilic reagents in various media. Thus, acylation of 56 by warming with glacial AcOH for short time yielded 3-N-acylamino-derivative 57, while aroylation of 56 via warming with 3,5-dinitrobenzoyl chloride in DMF produced the benzamido derivative 58. Refluxing 56 with PPh3 (similarly as Wittigs reaction) afforded the phosphiimino-derivative 59 (Scheme 21).
Most of alkylated amino-1,2,4-triazino [5,6-b] indoles obtained exhibit a wide range of biological activities [31] [32] . Similarly, treatment of compound 56 with MeI (1%aq.KOH. stiring at R.T), monochloroacetic acid (DMF), or chloroacetonitrile (DMF) lead to the direct formation of 3-N-alkyl derivatives 60-62 (Scheme 22) [30] .
Thiazolidin-4-one derivatives obtained exhibit a highly biological, pharma-cological, and medicinal activities [33] [35] . Thus, condensation of compound 56 with 4-chlorobenzaldehyde in AcOH under refluxing yield the Schiff base 63, which upon cycloaddition with thiolactic acid in refluxing 1,4-dioxane, afforded 2,3,5-trisubstituted thiazolidine-4-one (46) (Scheme 23) [30] . The formation of 64 is shown in Figure 12.
The former structure of 64 deduced from the correct elemental analysis and spectral measurements. The mass fragmentation pattern of 64 gives us a good indication about that stability Figure 13 [30] .
![]()
Scheme 21. Formation of compounds 57, 58 and 59.
![]()
Scheme 22. Formation of compounds 60-62.
![]()
Scheme 23. Formation of compounds 63 and 64.
![]()
Figure 11. Some important medicinal compounds.
It is interesting that the interaction between 3-amino-8-fluoro-5H-1,2,4-triazino [5,6-b]indole (56) and chloroacetonitrile in refluxing DMF lead to the direct formation of 3-amino-7-fluoro-10H-imidazo [3,2-b] [1,2,4]triazino [5,6-b]indole (65) (Scheme 24) [30] . Formation of 65 may be a simple nuclophilic attack of NH2 to more E+ center followed by cycloaddition reaction (Figure 14) [30] . Similarly, acylation and aroylation of compound 65 under the normal condition (RCOOH and ArCOX) produced the 3-N-acyl/aroyl amino-imidazo [3,2-b] [1,2,4]triazino [5,6-b]indoles 66 and 67, respectively (Scheme 24) [30] .
Finally, alkylation of 65 via treatment with MeI in aq.KOH at room temperature and/ or with chloroacetic acid in refluxing DMF yielded 3-N-alkylamino-deravitives 68 and 69, respectively (Scheme 25). Decarboxylation of 69 by warming with aq.KOH gave 3-methylamino-imidazo [3,2-b]-1,2,4-triazino [5,6-b]indole (68). The reaction of 65 with PPh3 in CH3CN yielded the phosphiimino 70 derivative (Scheme 25) [30] . The formation of compound 70 is indicate in Figure 15 [30] .
![]()
Figure 15. A possible route to formation of compound 70 from reaction of 65 with PPh3 in CH3CN.
![]()
Scheme 24. Formation of compounds 66 and 67.
![]()
Scheme 25. Formation of compounds 68-70.
The introduction of fluorine atoms to 1,2,4-triazine derivatives often enhance and improve those properties, especially the medicinal and pharmacological field [1] [31] - [36] . Recently, the high resistance of microbes towards most drugs and antibiotics, is driving an urgent need for the synthesis of new highly bioactive systems in view of control on these resistant [37] [38] [39] . Thus, all synthesized fluorine compounds 56-69 evaluated as anti-inflammatory agents by using the standard indomethacin drug as standards, according the reported method [30] , the activities were ranked as 65 > 56 > 64 > > 58 > 67 > 60. Both the compounds 56 and 65 which contains a fluorine atom and an amino-group at the end of presence systems form a type of bio-conjugated systems. Also, a higher activity of compound 64 may be the formation of type combination between the thiazolidine-4-ones, and fluorine, chlorine bonded the terminal of systems [30] .
6. Important and Applications
Fluorine containing 1,2,4-triazine moieties are high biological activity. Thus, the introduction of fluorine atoms to isolated, fused, and condensed 1,2,4-triazine systems can produce new bioactive targets depend on the position and magnitudes of total change. Most fluorinated 3-amino- or 3,5-diamino-6-aryl-1,2,4-triazines exhibit an anti-inflammatory activity exceeding that of lamotrigine drug as Figure 16 [40] .
![]()
Figure 16. Some important medicinal compounds.
Most of fluorinated 1,2,4-triazin-5-one moieties display anti-HIV activity [1] [5] . Also, some fluorine-substituted with phosphorphous-1,2,4-triazines exhibited an important antioxidant activity [8] . As well as mostly, fluorine substituted 3-thioxo-1,2,4-triazinones showed a potential inhibitor as cyclin depend kinas (CDK2) for tumor cell damage [5] [6] . Recently, some fluorinated fused and isolated 1,2,4-triazines systems have been reported to have biocidal affect as molluscicidal agents against some snails [24] . In addition, many new fluorine compounds substituted by some or more heterobicyclic moieties, especially 1,2,4-triazine moiety, showed a wide range of antimicrobial activity [1] [7] [9] [10] [16] [18] [33] . Finally, some fluorinated 1,2,4-triazinones synthesized use as enzymatic affects towered some fungi [2] [9] [13] [16] [25] .
7. Conclusion
A series of various fluorine compounds substituted with 1,2,4-triazine moieties have been developed by various routes. The results of these targets were characterized physically, chemically, or both, together with evaluations of the pharmacological activities. The introduction of fluorine atoms to heterocyclic nitrogen systems mostly enhances and improves the physical, chemical, and biological properties. In view of the fluorinated 1,2,4-triazine derivatives obtained, most have potentially beneficial applications for our life to treat various diseases such as anti-inflammatory, antimicrobial, or anti-HIV1 agents, or as cyclin dependent kinase inhibitors for tumor cell damage of DNA moiety. Hopefully, the present overview contributes an explanation of how new fluorine compounds bearing 1,2,4-triazine moieties and the related hetero-polycyclic nitrogen systems are formed and used.