1. Introduction
Zeolite is a word meaning “Fusing Rock”. It earned this name because of its ability to explode and disintegrate when it is heated. Zeolite was first discovered in 1856 by Swedish mineralogist Freiherr Axel Fredrick Cronstedt. Zeolites are a mineral group that is formed by the change of volcanic ash in aquatic environment millions of years ago, comprising alkali and alkaline-earth hydrated natural silicates. Zeolitization is a process in which feldspar and other aluminosilicates turn into zeolites. Zeolite minerals are in an aluminosilicate structure which is comprised of connected tetrahedral AlO4 and SiO4 via sharing oxygen atoms and can grow infinitely as a three dimensional web. Their structure is similar to honey combs or cages and includes alterable cations and water. Micro foramens between the units merge in micro windows and form one, two or three dimensional clearance systems and canals. The amount of clearance is between 20% and 50% of the total volume. These channels and cages are adequate for adsorbing molecules smaller than these channels and/or tunnels, so in this regard, zeolites are known as “molecular sieves” [1] [2] [3] .
One of the main properties of zeolites is its canals (pores), which have a homogenous structure in crystal form. Zeolites have ion exchange, absorption, molecular sifting and catalytical effect mechanisms because of these [4] [5] [6] [7] [8] . Zeolite skeleton is formed by the open crystal cage wave formation of different shapes of SiO4 and AlO4 tetrahedras. Its micro canal structure is formed by its crystal cage structure, which contains canals on a molecular scale [6] [7] .
There are more than 40 known minerals. The most important of these are clinoptilolite, heulandite and chabazite. With its chemical formula of (Na,K)6(Al6Si30O72)∙20H2O, clinoptilolite’s crystal system is monoclinic, and its competence in silicates class is 3.5 - 4, and its specific gravity is 2.2. It is vitreous, neat, transparent or semi-transparent. It can be colourless, white, pink, yellow or have a reddish hue. It is resistant to heat. Its Si/Al mol ratio is 0.425/5.25. It is a resistant zeolite to acid (pH: 1.5 - 11). Its crystal clearance is 39%. Its most important feature is its canal shaped clearance structures. Its water, oil and gas absorption capacity is relatively high. Clinoptilolite is formed via the transformation of volcanic glass and tuff into crystal materials with the effect of heat. Volcanic rocks form with basalt, rhyolite and andesite and with borate minerals in deep seas as residuum.
Clinoptilolite and heulandite are very similar in terms of their crystal structure and they cannot be distinguished from each other, even in X-Ray images. Both zeolite minerals are formed in a layered structure, tectosilicate sub class and their oxygen atoms are covered by silica or aluminium ions. Layers are formed by 8 - 10 edged open rings and layers are piled together and form canals and clearance within the crystal. Canals work like a molecular sieve because of their ability to allow certain molecules and deny certain others according to their sizes. Clinoptilolite’s difference from heulandite is its relative richness of silica and the fact that it is more resistant to heat. For example, heulandite transforms to another phase which is named as heulandite B in 230˚C and becomes completely amorphous in 350˚C. Clinoptilolite retains its crystal formation up to 700˚C. It is heulandite if the (Na + K)/(Na + K + Mg + Ca) ratio is bigger than 0.5; otherwise it is clinoptilolite [9] [10] ; or it is clinoptilolite if the (Na + K)/(Mg + Ca) ratio is bigger than 0.69; otherwise it is heulandite [11] . It is clinoptilolite if the Si/Al ratio per cell is bigger than 4; otherwise it is heulandite [12] .
Manisa-Gordes zeolites are in the structure of potassium, calcium clinoptilotite in high thermal stability and have a specific gravity between 1.98 - 2.18 g/cm3. Bulk density varies according to granule bulk between 0.693 - 1.22 g/cm3. The specific surface area of Gordes zeolites is 40.80 m2/g. Gordes zeolites release their foramen water in 50˚C - 90˚C, bound-water at 90˚C - 320˚C, crystal water at 360˚C - 770˚C and their structure begins to dissolve at 839˚C. Thermal stability endures up to 1000˚C. The water absorption capacity of Gordes zeolites is between 15% - 30%. Gordes zeolites have high thermal stability and their natural unit bulk weight is 1.36 g/cm3. Rock form Gordes zeolites has 0.418 - 0.477 W/m˚C of transmission coefficient. Meanwhile with the classified granule size, this changes to 0.098 - 0.273 W/m˚C. In mercury and vanadium adsorption studies, 1 gram of zeolite absorbed 0.310 mg vanadium and 2.841 mg mercury [13] . The structural features of Gordes clinoptilolites are presented in Table 1.
2. Geology of Manisa-Gordes Region
Turkey has one of the largest and richest reserves of zeolite in the world, including the Bigadic and Gordes reserves [11] [15] . Zeolite mineral formation is observed in nearly 2/3 of the tuffs in Miocene piles in the Manisa-Gordes region. 80% of these tuffs are comprised of heulandite and clinoptilolite. These tuffs are located in the proximity Gordes county centre and they are placed as two levels lithostratigraphically. According to this, they are comprised of a Miocene residuum pile with a thickness of nearly 1000 m, with one alluvial series at the bottom (bottom crude and fine grain units, blockstone-pebblestone sandstone and sandstone-pebble sandstone-local mudstone and shale) and a volcanoclastical lacustrine series at the top, which are inharmonious with the crystalline rocks of Menderes Massive and rock units of Izmir-Ankara Zone. Within this top series, at the bottom and the top (with 80 and 70 m of thickness respectively) are located two tuff series and an intercalated unit that is comprised of sandy, clayey, carbonated, tuffed and mixture of all of these. The tuff level possesses a rhyolite-rhyodacite character, the bottom tuffs are vitreous-crystal-partially lytic and lapilli ash-dust tuff arrayed from north to south. Top tuffs are vitreous-crystal, ash-dust tuffs. Phenocrystals are quartz, plagioclase (albite-oligoclase), sanidine
![]()
Table 1. Structural features of Gordes clinoptilolites [13] [14] .
and a low amount of biotites. Tuffs’ authigenic minerals within the field are zeolites (mostly heulandite, clinoptilotes, locally analcime and rarely philippsite), silica minerals (opal-CT and quartz), clay minerals (smectites and 10 A clays; illitseladonite), K-feldspar and carbonate minerals (calcite and rarely dolomite). Diagenesis of volcanoclastical series have caused a widespread zeolitization and particularly heulandites-clinoptilolite type group minerals have developed in particularly tuffs and their glass parts, clearance, pumis fibre and all connective material. There are two types of group minerals, mainly clinoptilolite in the bottom tuffs and fully heulandite in the top tuffs [11] [16] .
Experiments subject to this study are carried out upon the zeolite samples that were obtained from zeolite facilities still active in production in the Manisa- Gordes region. The Manisa-Gordes location map is illustrated in Figure 1.
![]()
Figure 1. Turkey-Manisa-Gordes location map.
Belonging to these fields in the research study, zeolite was taken from this field according to the method.
3. Utilization Areas of Zeolites
The areas of usage for zeolites can be described under four main headings, which are pollution control, the energy sector, agriculture and stockbreeding. Applica- tions for pollution control are; marginalizing heavy metals such as Pb, Cu, Zn, Cd and Hg from industrial waste waters, marginalizing ammonium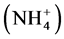 , which has a toxic effect, from city waste waters and drinking water, lowering water hardness, regulating pH-conductivity and raising the quality of drinking water, purifying chimney gases, cleaning up oil leaks, binding air pollutant gases such as SO2, CO, CO2, H2S, NH3, NOx, garbage rubbish disposal areas, metallurgy applications, and marginalizing radioactive materials such as Cs, Sr, Rb from nuclear wastes. Zeolites’ ion exchange, adsorption and molecular sieving properties are used for those applications. Applications regarding the naturalising and drying of natural gases, heat storage, oxygen generating, processing coal gases, separating industrial gas compounds such as CH4/N2 and N2/H2, oil refinement, and sun energy storage systems can be performed with the help of zeolites’ ion exchange, adsorption, molecular sieving and catalyst properties [17] [18] .
, which has a toxic effect, from city waste waters and drinking water, lowering water hardness, regulating pH-conductivity and raising the quality of drinking water, purifying chimney gases, cleaning up oil leaks, binding air pollutant gases such as SO2, CO, CO2, H2S, NH3, NOx, garbage rubbish disposal areas, metallurgy applications, and marginalizing radioactive materials such as Cs, Sr, Rb from nuclear wastes. Zeolites’ ion exchange, adsorption and molecular sieving properties are used for those applications. Applications regarding the naturalising and drying of natural gases, heat storage, oxygen generating, processing coal gases, separating industrial gas compounds such as CH4/N2 and N2/H2, oil refinement, and sun energy storage systems can be performed with the help of zeolites’ ion exchange, adsorption, molecular sieving and catalyst properties [17] [18] .
In agriculture and stockbreeding, zeolite, with its ion exchange, adsorption and molecular sieving properties, can be used as a fertiliser additive, in reclaiming soil by extracting excess water, conditioning soil for agriculture, preventing washing off of plant nutrients, stabilising pH in agricultural soil and regulating soil, as a carrier material for pesticides, moisture and insect control in grain stores, preventing ripening and hardening in fertilisers during storage, trans- ferring nutrient ions on agricultural grounds, binding cations of unwanted heavy metals such as Pb, Cd, Zn, and Cu, as additive for animal feed, in cleaning ponds in fish farms in order to provide enough oxygen, preventing effluvia in barns, as cat soil, strengthening bones, enhancing egg and bone development, and bone meal applications.
In addition zeolite, with its ion exchange, adsorption and catalyst properties, is known to be used as a filling material in paper production, for seeking uranium beds in mining, as a light component element and cement additive in construction, in water culture applications, powder detergent applications, as a defroster on highways and in many other industries such as medicine [17] [18] [19] [20] [21] .
4. Applications Technologic Tests of Zeolite
According to the work performed in taking into consideration the firing position and the colors; the zeolite sample shows us that it is unsuitable for use in the ceramic industry. Ceramic pre technological reviews on a zeolite sample are presented in Table 2.
The oil absorption test is made according to standard TS-2583 EN ISO 787-5/ December 1997 “The general test methods for pigments and fillers materials - Part 5: Determination of the value of oil absorption” and the value of oil absorp- tion is found to be 62 ml/100 g.
Analysis of Whiteness is made using a “Minolta Chroma Meter CR300” device. Examination of the zeolite sample as cat litter is made according to standard TS-12131/February 1997. The standard used in the production of sepio- lite-cat litter. The water absorption capacity is found as 64% and it has no mudding down and softening feature. Since the values of water absorption is less than 75%, it is unsuitable for use as cat litter. The bleaching on the original form and activated form are determined as 1.12 g sample/g tonsil, 1.08 g sample/g tonsil respectively. Because samples in pieces with weighs are larger than 350 grams decompose in water, density and water absorption tests were made according to TS 699/January 1987. According to the “ASTM C493-98 Standard Test Method for Bulk Density and Porosity of Granular Refractory Materials by Mercury Displacement” the standard, porosity value of the zeolite sample has been identified as 1.19 gr/cm3.
![]()
Table 2. Ceramic pre technological reviews on zeolite sample.
Because of the physical properties of the zeolite sample, the real density analysis could not be identified using device Accupyc Pycnometer 1330 Hc. In addition, water absorption tests could not be completed, due to the dispersion in water. The all of these results are presented in Table 3.
Determination of the original sample bleaching capability: After the sample was dried on 55˚C ± 5˚C until constant mass, it was ground to pass through the 200 mesh (74 micron) bottom sieve. 1 g of the sample was put into the tube, 20 ml of oil-benzene mixture was added, and it was shaken for 10 minutes. After 24 hours, the value of % T (permittivity) was read on a calorimeter and the equivalence of tonsil was determined.
Determination of the activated sample bleaching capability: After the sample was dried on 55˚C ± 5˚C until constant mass, it was ground to pass through the 200 mesh (74 mikron) bottom sieve. 100 ml of distilled water was placed in accordance beaker and 20 g of the sample was added. This mixture was then mixed thoroughly with begets and 12.5 ml, 98% concentrated H2SO4 was added. It was kept for 1.5 hours on a hot plate at boiling point. After the boiling time, the sample was left for 24 hours. Afterwards, the sample under vacuum was filtered until there was no acid in permeate and left to dry. The sample in the form of a dried cake was ground again and the bleaching process was applied to the original sample again. Chemical analysis results of the sample are presented in Table 4.
![]()
Table 3. Technologic tests results of zeolite.
![]()
Table 4. Chemical analysis results of the sample.
5. X-Ray Analyses of the Zeolite Sample
The zeolite sample was subjected to X-Ray diffractometer studies. Which diffraction distribution corresponds to which 2θ was defined by the peaks in the continuous spectrum by the X-Ray diffractometer shoots. The DHKL distances and reflection intensities which corresponds to each 2θ that provides Bragg diffraction condition (nλ = 2dSinθ) were detected from catalogues. In order to define the phases that the peaks belong to, diffraction indexes created by American Society for Testing Material (ASTM) were used. Those standardised cards were used to define the phases that have a crystalline formation in the samples. X-Ray diffractometer parameters used in the experiments are Heulandite, Clinoptilolite as diffraction.
In the X-Ray studies defined according to the conditions above, zeolite has the same properties and includes both Heulandite and Clinoptilolite. In Table 5, the detected mineral structure according to the X-Ray analysis is provided. Heulandite properties are higher than Clinoptilolite in the zeolite sample. In addition, quartz, feldspar and biotite minerals were also detected with X-Ray.
The result of XRD analysis carried out between 2˚ - 70˚ by a Philips PW 3710/1830 XRD analyser with a Cu X-ray tube is presented below in Figure 2. There are zeolite group minerals (heulandite-clinoptiolite, very little analcime ASTM No: 41 - 1478), feldspar group mineral, very little mica-group minerals, very little group of mixed layer clay minerals, very little amphibole group mineral, very little quartz (ASTM No: 33 - 1161), very little kaolin group clay mineral, very little calcite (ASTM No: 5 - 585), amorphous material in this zeolite sample.
6. Microscopic Analysis of the Zeolite Sample
Analysis results of the experimental subject of zeolite samples (mass and grain)
![]()
Table 5. X-Ray diffractometer parameters.
by Olympus SZx16 type binocular microscope are presented below. Microscopic images of zeolite sample can be seen in Figures 3(a)-(e) Zeolite’s binocular microscopic images, as can be seen in Figure 3(a), show the base surface under white clean zeolite minerals and partial biotite (mica) and ferrous oxide contamination can be observed (5×).
Zeolite’s binocular microscopic images, as can be seen in Figure 3(b), show ferrous oxide introvisions, in other words, entries with the sizes of approximately 959.99 μm - 589.74 μm into the zeolite (5×). Zeolite is processed in usage as either (+) or (−), depending on the desire for iron or not. Only in the very high quality zeolite productions, washing or acid cleaning operations can be possible under these sizes.
Zeolite’s binocular microscopic images, as can be seen in Figure 3(c), under microscopic visual clean zeolite grains are observed as 95% in the size of approximately 0.99 × 1.52 mm of broken zeolite sample (2.5×). However, the grain is observed and imaged as clamped together as biotite grain with the size of 200 μm.
Zeolite’s binocular microscopic images in the grain size of −1.5 mm, as can be seen in Figure 3(d) in the observation in which the zeolite’s broken grain material under the size of 1 × 1.5 mm was bated under the microscope, it was seen that besides the free zeolite grains, ferrous oxide mineral daubing (yellowish, reddish) and partially free and mostly pointed clamped biotite grains were
observed gravimetrically under the R04 visuals of nearly 20% (1.5×). This event points out that the zeolite is contaminated. According to the usage areas, this type of zeolite can be selectively broken under 1 mm or 0.5 mm and converted to a cleaner product via enrichment according to density. Waste and side products that emerged after this enrichment process can also be used according to the consumption, for example in soil improvement.
Zeolite’s binocular microscopic images in the grain size of −1.5 mm, as can be seen in Figure 3(c). is bated into the zeolite sample, it is seen and observed that quartz and ferrous oxide and other biotite-clamped grains are intensified because they are denser than zeolite, and if a clean process is performed among them, for example planar attraction, cleaner crystal quartz can be obtained (1.25×). It is measured with a digital camera that mentioned crystal quartz is in the size of 433 - 373 μ, tracked by RO5. If this sample A is selectively and protectively disintegrated under 400 μ, for example subjected to dry or damp density dependent enrichment, followed by magnetic enrichment, it is observed that clean crystal quartz can be obtained. These obtained quartzes can be used in the glass or ceramic sector.
Images obtained by the microscopic analyses carried out on the thin sections prepared from a zeolite sample are presented in Figure 3(f). According to these images, the zeolite sample contains trace minerals such as biotite, metamorphic rock remnants, iron, quartz, plagioclase (feldspar), as seen in Figure 3(f).
In the microscopic analyses, the first degree of the softest zeolite minerals and clay show fine grain sized collapse, third degree quartz are in the largest sizes, the middle degree solid biotite and ferrous oxide are in the second degree. If extremely thin sized material is required, the surface of the quartz below 500 μ will be free and biotites will leave the surface. If 99% of mineral purity, high quality zeolite is required, it is concluded that the iron, hematite and biotites, which are present in trace amounts within the structure, can be removed by magnetic separation. In the broken grain material below the 1 × 1.5 mm zeolite sample size, ferrous oxide mineral daubing (yellowish, reddish) and partially free and mostly pointed clamped biotite grains are observed. This shows that the zeolite is contaminated. According to the request of the usage fields, this type of zeolite can be selectively disintegrated below 1 mm or 0.5 mm and can be subjected to density-dependent enrichment in order to obtain a cleaner product. It is observed and imaged that, the zeolite sample has more intense quartz and ferrous oxide and grains clamped with other biotite, compared to zeolite, and it is possible via a clean process, for example surface attraction to obtain cleaner crystal quartz. Crystal quartzes are in the sizes of 433 - 373 μ and it is observed that if the zeolite sample is subjected to selective and protective disintegration under 400 μ and enriched according to dry or damp density, followed by a magnetic enrichment, clean crystal quartzes can be obtained. The obtained quartz can be used in the glass or ceramic sector. It is concluded that zeolite mineral is relatively clean, but for very high quality production, it should be downsized to below 150 μ and the contaminating minerals must go free and with a dry or damp environment enrichment, it is possible to obtain a multi quality zeolite concentrate. It has been proven with microscopic analysis and in digital media that clean zeolite grains mostly go free in a wide spectrum from 600 μ to 300 μ and even thinner. If it is milled down to these sizes and enriched in a dry and damp environment quality zeolite concentrate production will be possible. Within the scope of this study, according to the microscopic analyses results carried out towards the assessment of the structural features of Manisa Gordes zeolites, it should be considered that structural contaminant gang minerals must be removed and quality zeolite should be obtained with ore preparation and enrichment methods. Based on the ore formation, realisation of selective mining should be taken into consideration in zeolite production. Environmental contamination should be avoided and preventive production measures should be taken on this matter (for example: dust suppression curtains should be used.
Morphological analyses were carried out using SEM. SEM observations showed the presence of micro-particles in the shape of smooth surface. Displays of an electron microscopy of zeolite minerals are shown in Figure 4.
7. Conclusions
In Turkey, clinoptilolites are generally used in agriculture and stockbreeding. The clinoptilolite group of zeolites with 85% purity has many areas of usage, according to their size. In this case, products gathered from primary crushing
can be used in gas purification. Products under 5 mm and gathered from secondary crushing can be used in water filtering (filtration) or put into use as animal mat or fertiliser additive. These processes generally use zeolites of 2 - 5 mm. The zeolites used in this study should be used for industrial areas and the improvement of agricultural lands. Zeolites that show efficiency in agricultural production and are needed to produce economic efficiency in water and fertiliser are carried out using consciously. Especially in Turkey, soil pollution created by immoderate use of water and fertiliser is eliminated by the use of zeolites. They are used in the prevention of heavy metal pollution such as Pb, Cu, Zn, Cd and Hg, which pollute the environment via industrial wastes in solid, liquid, gas. The use of zeolite via high adsorption force will contribute to preventing toxic contamination created by heavy traffic caused by heavy metal gases (Pb, Cu, Zn, Cd and Hg) nowadays and achieve a healthy environment for both people and cities. Therefore, Gördes zeolites should be considered for this purpose. The investigations indicate that it is also used as an additive for cement, which is more economical with lightweight structural elements in the construction industry, which can be done with zeolite. According to the results of microscopic examination in order to identify the structural features of Manisa Gordes zeolite, removing polluting gangue minerals in the structure and production of more quality zeolite provided by mineral processing and beneficiation methods should be considered. Based on the formation of ore, selective mining for production of zeolite should be considered. We should avoid environmental pollution and precautions should be taken for this required production (for instance, using a dust holding curtain).