The Effects of Fe2+ on the Aggregation Behavior of Residual Hydrophobic Modified Polyacryamide ()
1. Introduction
Polymer flooding plays a major role in global crude oil recovery [1], especially in China [2] [3]. Usually, there was residual polymer in treated oily wastewater produced from polymer flooding (TOWPF). TOWPF can be used for reinjection or polymer flooding [4] [5]. In addition, there were many other substances in the TOWPF, such as emulsified oil, suspended solid, Fe3+, Fe2+, cationic water clarifier and SRB bacteria so on. These substances have great influence on thestability of residual polymer, especially for Fe2+. If the polymer is not stable, then it might aggregate and sedimentate in the buffer vessel, thereby affecting the reinjection process. Therefore, it’s necessary to investigate the aggregation behavior of residual polymer in water. Currently, lots of literatures have reported on the influences of temperature and concentration of salt on the aggregation behavior of the polymer used in polymer flooding in water [6] [7] [8] [9]. However, there is no reports found to discuss on the effect of Fe2+ on the aggregation behavior of the polymer produced in polymer flooding in water. Generally, Dynamic Scattering Light (DSL) and fluorescent microscopy method [8] [9] are most frequently used to study the aggregation behavior of polymer in water. In this paper, DSL and fluorescent microscopy were used to investigate the effects of Fe2+ on the aggregation behavior of residual polymer systematically, and the results are expected to be applied in the analysis for the stability of residual HMPAM in TOWPF.
2. Experiments
2.1. Materials and Instruments
TOWPF was taken from an offshore oilfield in china. In this offshore oilfield, oily wastewater treatment process is shown in Figure 1.
The oil displacement agent was hydrophobic modified polyacryamide (HMPAM). Therefore, there was residual HMPAM in the produced wastewater. The produced wastewater containing HMPAM was treated by skimmer, air floatation and walnut shell filter in turn and TOWPF was obtained [10] and came to buffer vessel. Its quality is listed in Table 1. In general, the TOWPF would be stay in buffer vessel for about 2 - 48 h. However, during this period, precipitates containing HMPAM would appear at the bottom of buffer vessel [11] [12]. This phenomenon represented that the stability of residual HMPAM in TOWPF was destroyed by some reason.
Pyrene was received from Aladdin-Reagent Corporation (Shanghai, China).
Fluorescence spectrum was measured by LS55 fluorescence spectrophotometer (PerkinElmer Corporation, England).
Dynamic light scattering (DLS) experiments were performed using Brookhaven Instruments 90 Plus/BI-MAS (Brookhaven Instruments, NY, USA). Hydrodynamic radius (Rh) was investigated by the DLS and calculated according to the CONTIN algorithm.
Size exclusion chromatography (SEC) analysis was conducted using a Water 515 liquid chromatograph instrument connected with a Waters 2410 refractive index detector at 30˚C. The gel permeation column was linear ultrahydrogel (7.8 × 300 mm2), and the
![]()
Table 1. The composition of simulation formation (mg/L).
![]()
Figure 1. The oily wastewater treatment process in one offshore oilfield in China.
solvent used was distilled water. Polymer standards of Dextran were used from NICPBP (National Institute for the Control of Pharmaceutical and Biological Products). The samples were dissolved in 0.1 M NaCl solution and analyzed at a flow rate of 0.8 mL∙min−1. Number-average molecular weight (Mn), weight-average molecular weight (Mw) and polydispersity index (PDI) were measured by SEC.
Intrinsic viscosities ([η]) of polymers were measured with the “five-spot”dilution method using an Ubbelohde viscometer at 30˚C, where the solvent was 1 M NaCl solution. Viscosity-average molecular weight (Mw) was calculated as follows [13]:
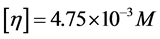
2.2. Extraction of Residual HMPAM in TOWPF
Firstly, 2 L TOWPF was concentrated to 200 mL. Then, the concentrated TOWPF was purified by osmotic membrane with molecular weight cut off 10,000 for three days. At last, after vacuum drying for 8 h under the condition of 60˚C, the HMPAM in TOWPF was obtained. The structure of HMPAM is shown in Figure 2.
2.3. Aggregation Behavior Study of Residual HMPAM
2.3.1. Preparation of Residual HMPAM Solution Containing Pyrene
Firstly, 1 L HMPAM aqueous solution (60 mg/L) was prepared by using simulation formation water as solvent (the composition of simulation formation is listed in Table 2). Then, 5 mL solution of pyrene in ethanol (concentration of pyrene was 500 mg/L) was added into the HMPAM aqueous solution. At last, the mixture was allowed to heat at 65˚C for 2 h to remove the ethanol and HMPAM solution containing pyrene was obtained.
2.3.2. Fe2+ Mixed with Residual HMPAM Solution Containing Pyrene
Firstly, the substance which was listed in Table 2, such as oil, suspended solid, Fe3+, and so on, was added respectively to 800 mL HMPAM solution containing pyrene (the dosage of substance was referred to Table 1).
![]()
Table 2. The characterizations of residual HMPAM.
Then, the mixture was stirred by an emulsifying machine at 7000 r/min for 15 min. At last, the mixture was distributed into four tubualted bottles (see Figure 3) for the next experiment.
2.3.3. Fluorescence Spectrum and DLS
Firstly, four tubualted bottles were kept at 65˚C for 0 h, 8 h, 24 h and 48 h, respectively. Then, different samples were obtained successively from the bottom of tubualted bottle. The first 50 mL was the bottom solution, the intermediate 100 mL was the middle solution and the last 50 mL was the top solution.
The solutions at different layers were filtered by 0.8 μm microporous membrane and then the aggregation behavior of residual HMPAM was investigated by the fluorescence spectrum and DLS.
Because hydrophobic groups in HMPAM were not water-soluble, they would aggregate and hydrophobic domain can be formed by hydrophobic groups from one HMPAM molecular or several HMPAM moleculars [14] [15]. Pyrene would be soluble in the hydrophobic domain. The vibronic structure of the fluorescence spectrum of the monomeric pyrene is known to be sensitive to the local polarity. In particular, the raito I1/I3 of the intensities of the first and third vibronic peaks increases on going from aliphatic to polar solvents [16] [17] [18], and can be used as an index of the effective local polarity of the pyrenesolutilization site in the hydrophobic domain. The raito I1/I3 was calculated as the ratio of the intensity of peak I1 (374.0 nm) to that of peak I3 (385.0 nm) of the vibration fine structure of pyrene monomer emission. In addition, usually, the I1 or I3would increase with number of hydrophobic domain because more pyrene can be soluble in the solution when there were more hydrophobic domains. Therefore, the value of I1 can reflect the number of hydrophobic domain at the same experimental condition. For DLS, the Rh obtained by DLS can reflect the size of HMPAM aggregates.
3. Results and Discussion
3.1. Characterization of Residual HMPAM
The characterization of residual HMPAM is listed in Table 2. It can be found that its molecular weight was not high, but its polydispersity was high. Because its molecular weight was not high, its mobility may be similar with the small molecule surfactant. There were intermolecular aggregation for residual HMPAM.
![]()
Figure 3. The picture of tubualted bottle.
3.2. Fluorescence Spectrums
Figure 4 shows the fluorescence spectrums of blank residual HMPAM solution at different layers and different times. The fluorescence spectrums of blank residual HMPAM solution at different layers were very close at the same time. As time increasing, the fluorescence intensity increased. Table 3 lists the I1/I3 of blank residual HMPAM solution at different layers and I1,t/I1.0h at different times. The results showed that the I1/I3 of blank residual HMPAM solution at different layers were closed and were about 0.85. Interestingly, the I1/I3 was not influenced by time. This phenomenon presented that the polarities of hydrophobic domain formed by residual HMPAM at different layers of blank residual HMPAM solution were the same. Therefore, the blank residual HMPAM solution was homogeneous and the polarity of hydrophobic domain was not changed during 48 h. Meanwhile, the increasing I1,t/I1.0h represented that the number of hydrophobic domain could increase with time because the formation of hydrophobic domain was a slow process for blank residual HMPAM solution.
![]()
Figure 4. Fluorescence spectrums of blank residual HMPAM solution at different layers and different time.
![]()
Table 3. The I1/I3 of blank residual HMPAM solution at different layers and I1,t/I1,0h at different times.
Table 4 shows the influence of Fe2+ on the fluorescence spectrums of residual HMPAM solution at different layers and different times. After the addition of Fe2+ into residual HMPAM solution, the I1/I3 was much larger than that of blank and not influenced by time too. It showed that the polarity of hydrophobic domain was increased. The reason of Fe2+ for increasing I1/I3 may be the chelation between these substances and carboxyl group in HMPAM. After the chelation, the moving ability and rotation ability of HMPAM decreased and the hydrophobic group used to formation of hydrophobic domain was decreased (see Figure 5). Therefore, the polarity of hydrophobic domain increased.
In addition, as shown in Table 4, after the addition of Fe2+, the I1,t /I1,0h increased with time,. Compared to the I1.48h/I1.0h of blank HMPAM solution. It can be found that the addition of Fe2+, the HAPAM solution had much larger I1.48h/I1.0h than that of blank. It represented that the Fe2+ was helpful for the increase of hydrophobic domain. Usually, the increasing hydrophobic domain means the increasing aggregation degree. In summary, when there was Fe2+, HMPAM solution had the largest aggregation degree (I1.48h/I1.0h = 1.89); After the addition of Fe2+, the chelation between Fe2+ and carboxy group in HMPAM would increase with time during 48 h. Therefore, the number of hydrophobic domains increased with time obviously.
3.3. DLS Results
Usually, the increasing hydrophobic domain may be caused by increasing number of HMPAM aggregates or increasing size of HMPAM aggregates. The results of DLS can reflect that whether the size of HMPAM aggregate increased or not. Figure 6 and Table 5 showed the effects of Fe2+ on the relationship between Rh of HMPAM at different
![]()
Figure 5. The change of aggregation behavior of residual HMPAM after the addition of Fe2+.
![]()
Table 4. The influence of Fe2+ on the I1/I3 of residual HMPAM solution at different layers and different times.
![]()
Table 5. The influence of Fe2+on the Rh value of residual HMPAM solution at different layers and different times (nm).
time. For blank residual HMPAM solution, the Rh had no great change with time. When there was Fe2+ in solution, the Rh had no great change with time, too. The results showed that the increasing of I1,t/I1.0h was caused by the increasing number of HMPAM aggregate.
4. Conclusion
The influences of Fe2+ on the aggregation behavior of residual HMPAM in TOWPF were studied by fluorescence spectrum and DLS. The results showed that Fe2+ can cause the aggregation of HMPAM molecular chains and increase the number of polymer aggregates largely due to the chelation between carboxyl groups and Fe2+, which may result in precipitating in water and producing lots of oily sludge. Therefore, some chelating agent should be added into produced water contain residual HMPAM during the treatment process for the sake of shielding the influences of Fe2+.
Supported
This work was supported by Study on Reinjection Technology of Sewage with Polymer of Bohai Offshore Oilfield (Grant no. CNOOC-KJ 125 ZDXM 06 LTD NFGC 2014-01).