Adsorption Kinetics of Cupric Ions on Mixture of Modified Corn Stalk and Modified Tomato Waste ()
1. Introduction
Most heavy metal ions are deleterious in excessive concentrations. Heavy metal ions and their toxic effects have become a major world concern, causing harm to human life and the environment. This pollution is released to soil and water by human industrial activities and has considerable effects by joining the food chain [1] [2] . Various industries produce and discharge wastes containing different heavy metal ions into the environment. These include mining and smelting, surface finishing industry, energy and fuel production, fertilizer and pesticide industry, leatherworking, photography and electric appliance manufacturing. Thus, metals cause serious environmental pollution, threatening human health and ecosystem [3] .
The agricultural application of sewage sludge is limited by organic pollutants and heavy metal ions because they tend to accumulate along the food chain and bring potential risks to animals and humans [1] [2] .
According to the World Health Organization (WHO), U.S. Environmental Protection Agency (USEPA) and many other government environmental protection agencies, it has been set the Maximum Contaminant Levels (MCLs) for the heavy metals which exist in drinking water as well as trade effluent [4] . Industrial wastewater treatment containing low concentrations of pollutants is becoming increasingly important [5] [6] . Copper is one of the broadly used materials which cause neurotoxicity, jaundice and liver and kidney toxicity. It may be found as a dangerous contaminant in food, especially shellfish, liver, mushrooms, nuts and chocolate [7] [8] [9] . There are different methods for the removal of heavy metals including chemical precipitation, reverse osmosis, ion exchange, and coagulation [1] [10] [11] . However, the application of such methods is limited due to some reasons such as incomplete metal removal, energy- intensive, economically expensive, and generation of secondary waste products [12] [13] . Adsorption is the most efficient and versatile method for removing any contaminants like heavy metal, especially if accompanied with appropriate regeneration steps. This solves the problem of sludge disposal and changes the system to more economically viable, especially if low-cost adsorbents are used [13] [14] [15] . Agricultural by- products usually are composed of basic constituents like lignin and cellulose and may also include other polar functional groups of lignin, which includes alcohols, aldehydes, ketones, carboxylic, phenolic and ether groups. The advantage of these groups is the ability to bind heavy metal ions by donation of their electron pair to form complexes with the metal ions in solution [16] .
Corn is one of the largest products in the world. It includes by-products such as corn cob, corn husk, corn leaf and corn stalk which are abundant agriculture residues, but most are burnt without utilization [17] . In addition, there is another agricultural by- product, tomato waste, which is abundantly available in Iran. If an adsorbent is a by- product or waste material from another industry, is abundant in nature, or requires little processing, it can be assumed as “low cost” adsorbent [18] .
The main objective of this research was to study the mixture of modified corn stalk as mentioned in our previous research [1] and tomato waste to determine their adsorption capacity in removing Cu(II) ions from simulated contaminated samples. The adsorption equilibrium was expressed using Langmuir and Freundlich models. Also, kinetic parameters for removal of copper ions from wastewaters using corn stalk/tomato waste as an adsorbent were investigated via adsorption isotherms and kinetic models. Experimental parameters affecting the biosorption process such as pH, time and ion concentration of solution for Copper(II) removal were optimized.
2. Materials and Methods
2.1. Adsorbent Preparation
Raw corn stalk (RCS) and tomato waste were obtained from a farm near Shiraz, Iran. After removing the leaves from the stalk and separation of impurities from tomato waste, the biomasses were cut into small slices. The samples were then ground through a 70-mesh sieve to achieve a uniform particle size. Then each powder was separately washed three times with distilled water and was dried in an oven at 50˚C until it reaches a constant weight.
Leyva-Ramos [19] method was used for oxidation of the corn stalk and the tomato waste. Each of the biosorbents was oxidized separately with nitric acid solutions as a chemical activator by adding 20 g of each to 100 ml of the acid solution in a flask. The concentration of the acid solution was 1 M. The whole solution containing the corn stalk and the tomato waste was heated for 2 h keeping the temperature at 50˚C, inconsistent with the author’s previous work [1] . To prepare the modified corn stalk (MCS)/ Modified tomato waste (MTW) mixture, the solutions were added together drop by drop. The solution was mechanically stirred for 48 h to disperse the particles into each other homogeneously.
Afterward, the whole solution was allowed to cool and drained to separate the biosorbents from the solution. The mixture was washed several times with distilled water until the pH of the distilled water ceased to change during washing. It was finally dried in an oven at 50˚C for 24 h. At last, the biosorbent was stored in plastic bottles for further use.
2.2. Characterization of Adsorbents
In this work the pH at point zero charge (pHpzc) of the MCS/MTW mixture was determined by the pH drift method used by Yang et al. [5] , with the modification that sodium chloride was used as an inert electrolyte. Before measurement of pH drift, the biosorbent was completely washed with water followed by dilute sodium hydroxide (0.01 M) to neutralize any free nitric acid that may have remained. Finally, the adsorbent soaked in HCl for 24 h. After filtration, the ‘‘enriched’’ mixture was washed with distilled water, and it was then air-dried. This procedure was done to ensure the removal of any potential effects on pH drift due to the dissolution of salts in the MCS/ MTW mixture. The pH of test solutions was adjusted in 0.005 M NaCl using 0.5 M HCl or 0.5 M NaOH. A 0.06 g of the prepared biosorbent was measured and added to 20 mL of the pH-adjusted solution in a plastic capped vial and equilibrated for 24 h [5] . The final pH was measured using a pH meter (model 827-metrohm) and plotted against the initial pH. The pHPZC was found by the point that curve crosses the pHi = pHf line.
2.3. Batch Experiment and Analysis
The aqueous solution of heavy metal was prepared with dissolving CuSO4 in distilled water. The adsorption isotherm experiments were performed at 220 rpm on the magnetic stirrer with 1 g of adsorbent in Erlenmeyer flask containing 100 ml of CuSO4 solution with the initial concentration of 25, 50 and 100 mg/L at 298 K and pH = 2 - 6, as metal ions were expected to form metal hydroxide at pH values greater than 6 regarding to the chosen concentrations [20] . The pH of the solution was adjusted with adding 0.01 M nitric acid or potassium hydroxide. In order to determine the amount of Cu ions in the solution, an AA-800 atomic absorption spectrophotometer made by Perkin- Elmer Company was used with limit of detection (LOD) equal to 1 ppb (the detection limit (according to IUPAC) is the smallest concentration or absolute amount of analyte that has a signal significantly larger than the signal arising from a reagent blank).
2.4. Sorption Experiments
 (1)
(1)
where qe is the adsorption capacity per unit mass of the adsorbents (mg/g) while C0 is the initial concentration of the metal ion (mg/l) and Ce is the concentration of the metal ions after equilibrium, V and M are volume of the solution (l) and mass of adsorbent (g), respectively.
At predetermined intervals of time, solutions were analyzed for the final concentration of Cu2+. At specific time t (min), the amount of adsorption qt (mg/g), was calculated by Equation (2) [21] :
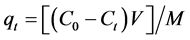 (2)
(2)
where Ct (mg∙l−1) is concentration of Cu2+ at time t.
2.5. Equilibrium Isotherms
Adsorption isotherm is used to indicate interaction of adsorbate with the adsorbent materials. It is necessary to establish the most suitable correlations for the equilibrium data of the system for optimizing the design of adsorption process to remove Cu(II) ion. The Langmuir and the Freundlich isotherms were used to correlating the equilibrium data for Copper(II) removal [13] .
One of the most important models of monolayer adsorption is Langmuir isotherm which is based on the assumption of a fixed number of adsorption sites, and each site can hold only one adsorbate molecule (the adsorbed layer is one molecule in thickness). All sites are same, and there is not any interaction between the adsorbed molecules [22] [23] .
The linearized form of Langmuir isotherm is (Equation (3)) [19] :
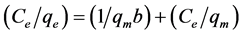 (3)
(3)
where qe is the amount of adsorbed ions per unit mass of adsorbent at equilibrium (mg/g adsorbent), Ce; the concentration of adsorbate remaining in the solution at equilibrium (mg/L), b; the constant related to adsorption net enthalpy, qm is the maximum adsorption capacity (mg/g). In addition, constants b and qm can be evaluated from the plot of Ce/qe versus Ce.
The dimensionless factor RL, called separation factor is another characteristic parameter of the Langmuir isotherm which estimates the degree of suitability and can be obtained using the following equation (Equation (4)) [7] [24] :
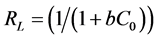 (4)
(4)
Suitability of the isotherm can be understood from RL. The values less than unity show that the sorption of metal ions to the adsorbents particles is optimum and the sorption process is favorable. Its value indicates the type of isotherm to be irreversible (RL = 0), favorable (0 < RL < 1), linear (RL = 1) or unfavorable (RL > 1).
The prepared solutions and adsorbents were left in contact with each other until equilibrium was reached. A sample was taken to determine the final Cu(II) concentration in different times at the intervals of the experiment, to evaluate the equilibrium time. After 2 h, the adsorbent was separated from the solution using a filter. The solution was then analyzed using an atomic absorption spectrophotometer to determine the equilibrium concentration of the Cu(II) ions.
Another important isotherm is Freundlich isotherm which proposes a monolayer sorption with a heterogeneous energetic distribution of active sites, accompanied by interactions between adsorbed molecules. Freundlich isothermis an empirical relation between the concentration of a solute on the surface of an adsorbent to the concentration of the solute in the liquid with which it is in contact. It can be expressed as Equation (5):
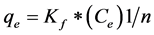 (5)
(5)
A logarithmic form can also be expressed as Equation (6):
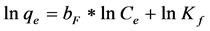 (6)
(6)
where Kf is the Freundlich constant and bF is the Freundlich exponent.
2.6. Sorption Kinetics
The two main types of sorption kinetic models, i.e., pseudo-first and pseudo-second- order models were considered in order to examine a suitable rate of adsorption and investigate the controlling mechanism of adsorption process [7] [25] .
Porous adsorbents adsorb any solute in four main stages: (1) transfer of solute from the bulk solution to the solution nearby the adsorbent, (2) transport of solute from surrounding surface of the adsorbents to the surface of adsorbent, (3) transfer of solute from adsorbent surface to the active internal sites, and finally (4) binding of the solute molecules to the available adsorption sites which present on the internal surfaces of the adsorbent [26] .
The pseudo-first-order reaction equation of Lagergren [7] [27] is vastly used for the adsorption of liquid/solid system based on the capacity of adsorbents. This model assumes that the rate of surface site’s change concentration is proportional to the amount of unoccupied surface sites which is remained [28] . This model can be expressed as Equation (7):
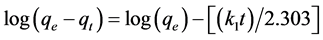 (7)
(7)
where qe and qt are the amounts of adsorbed metal ions per unit mass of biosorbent at the equilibrium (mg/g) and at time t, respectively and k1 is the Lagergren rate constant of the first-order adsorption (min−1).
The pseudo-second-order model is based on the assumption that the rate is proportional to the square of the number of remaining free surface sites [28] . The linearized second-order kinetic model is given by Equation (8):
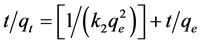 (8)
(8)
where k2 is the rate constant for the second-order adsorption kinetics (g/mg min).
3. Results and Discussion
3.1. Point of Zero Charge
The point of zero charge the pHPZC of any adsorbent is a very important characteristic that determines the pH at which the surface has net electrical neutrality [5] . The surface acidity and basicity are important criteria because it describes the surface chemistry of the adsorbents. The combined influence of all the functional groups of the adsorbents determines pHpzc [21] .
In this work, the pH drift method was employed to determine this parameter. As shown in Figure 1, the curve cuts the pHi = pHf line at 3.8.
According to the author’s previous research [1] , pHpzc of the present research was greater than the pHpzc of the Modified Corn Stalk (3.8 > 1.96). Therefore, this result revealed that the MCS has more acidic sites than the MCS/MTW mixture. Therefore, the optimum pH must be chosen greater than 4.5 which was found in the previous research.
![]()
Figure 1. Suspension test for determining the pH of point of zero charge of equal portions of modified corn stalk (MCS)/Modified Waste Tomato (MWT) mixture by pH drift method.
3.2. Effect of Solution pH
The pH of the aqueous solution has been reported to present a significant influence on the adsorptive uptake of Cu2+ ions due to its impact on both the surface binding-sites of the adsorbent and the ionization process of the metal ions [21] [29] [30] .
The pH of the point of zero charge measurement on these adsorbents supports this observation, below which the surface is net positively charged and unable to bind Cu2+ ions [5] . The effect of initial pH on adsorption was determined at different pH values (2 - 6). T he effects of initial pH on the adsorption of Cu(II) by the adsorbent mixture are presented in Figure 2.
At lower pH values (pH 2.0), the H3O+ ions are competed with the Cu(II) for the exchange site in the adsorbent [31] . Because H3O+ occupied the sites on adsorbents, heavy metal ions remained in solution. Another factor which restricts adsorption is that the hydroxyl group also combines easily with H3O+ ions or it has a reaction with functional groups. At high pH values (pH 6.0), the concentration of Cu(II) was higher than H3O+ ions, so that more exchange sites were provided for heavy metal ions [17] [29] . As shown in Figure 2, the highest capacity was reached at pH = 6. In addition, the pH of the solution decreased after Cu(II) binding, suggesting that with the binding of Cu(II) on the biomass, protons are released into the solution from the biomass.
Ion removal is highly concentration-dependent, the more concentrated the solution, the better the adsorption [17] [32] . Therefore, the solution concentration was chosen 100 mg/l to study the effect of pH.
3.3. Effect of Initial Concentration on Cu(II) Biosorption
Biosorption of Cu(II) ions by MCS/MTW mixture as a function of the initial metal ion concentration was presented in Figure 3 for 1 g/100ml biosorbent dosage with pH of 6. It was clear that copper biosorption capacity of MCS/MTW mixture increased as the initial metal ion concentration increased, while the removal efficiency of Cu(II)
![]()
Figure 2. Effect of pH on Copper(II) biosorption using equal portions of MCS/ MWT equal portion mixture particle.
![]()
Figure 3. Effect of initial Cu(II) concentration (pH: 6, biosorbent dosage: 1 g/100mL, temperature: 298 K, contact time: 120 min).
decreased. The concentration gradient of biosorption was the driving force to overcome mass transfer resistances between the adsorbent and adsorption medium which increased biosorption capacity. A drop in the removal efficiency of Cu(II) may be due to the saturation of surface area and active sites of adsorbent [13] [33] . The maximum uptake capacity of the adsorbent was obtained in the concentration of 100 mg/L, and it was reached to 4.64 mg/g.
3.4. Adsorption Equilibrium Studies
Langmuir constants of MCS/MTW mixture, qm (mg/g) and b (L/mg) were obtained from the slope and intercept of the linear plot of Ce/qe versus Ce (Figure 4), respectively. The Langmuir model correlation coefficient and constants were: R2 = 0.987, qm = 25 mg/g, b = 0.0039 L/mg. The RL values were found to be between 0.71 and 0.91, indicating that the biosorption of Copper(II) on MCS/MTW mixture was favorable (Figure 5).
Also with the linear plot of lnqe versus lnCe, Using the Freundlich isotherm model, the values of bF and KF were obtained and reported as, R2 = 0.998, n = 1.11 g/L, KF = 0.12 mg/g.
The sorption data for the investigated biosorbent showed a good fit with the Freundlich isotherm model and it had a better fit than the Langmuir isotherm model (0.987). It should be mentioned that both the Langmuir and Freundlich isotherms have good conformity. Figure 4 and Figure 6 show the Langmuir isotherm model and the Freundlich isotherm model, respectively, for sorption of Cu(II) ions onto these investigated biosorbents.
It was found that the adsorption capacity of modified corn stalk increased from 20.8 mg/g to 25 mg/g by using modified tomato waste as an adding biosorbent.
Furthermore, Cu(II) was favorably biosorbed by MCS/MTW mixture due to n value obtained from the Freundlich isotherm which was higher than 1.0 [13] [34] .
![]()
Figure 4. Langmuir isotherm model of Copper(II) biosorption onto equal portions of MCS/ MWT mixture particle.
![]()
Figure 5. Effect of Cu(II) initial concentration on dimensionless separation factor RL.
![]()
Figure 6. Freundlich isotherm of model of Copper(II) biosorption onto equal portions of MCS/MWT mixture particle.
3.5. Kinetic Studies
Sorption kinetic studies were conducted by adding 1 g of MCS/MTW mixture to 100 ml of Cu(II) solution with initial concentrations of 25, 50 and 100 mg/l and initial pH of 6. The solution was poured into a flask and placed in a shaking incubator at a speed of 220 rpm to measure the adsorption of MCS/MTW mixture at different time intervals at 298 K. The straight-line plots of log(qe − qt) against t and of t/qt against t were used to determine the rate constants and correlation coefficients for the first and second-order kinetic models, respectively (Figure 7 and Figure 8).
As observed, the higher values R2 (correlation coefficient) are resulted in the pseudo- second-order model in all the initial concentrations over the entire region (Figure 8). The pseudo-second order model demonstrated the best fit. In addition, as shown in Table 1 the first-order model failed to provide a realistic estimate of qe. On the other hand, the calculated qe based on the second-order model is in good accordance with the experimental one. This result showed that chemisorptions mechanism most likely controlled the biosorption of Cu(II) onto MCS/MTW mixture [13] .
![]()
Figure 7. Pseudo-first order rate constants for adsorption of the Cu(II) ions onto equal portions of MCS/MWT mixture (pH = 6, M = 1 g, T = 298 K, V = 100 mL).
![]()
Figure 8. Pseudo-second order rate constants for adsorption of the Cu(II) ions onto equal portions of MCS/MWT mixture (pH = 6, M = 1 g, T = 298 K, V = 100 mL).
![]()
Table 1. Pseudo-first and second order rate constants for adsorption of Cu(II) ions onto equal portions of MCS/MWT mixture (pH = 6, M = 1 g, V = 100 mL and T = 298 K).
4. Conclusion
This study reveals that sorption experiments of the Cu(II) ions from aqueous solutions onto a mixture of corn stalk and tomato waste which were oxidized with nitric acid were used successfully. The sorption studies indicate that metal ion uptake was pH- dependent and maximum biosorption of Copper(II) ion was obtained 4.54 mg/g at pH = 6. It was clear that the adsorption capacity of modified corn stalk increased from 20.8 mg/g to 25 mg/g by using modified tomato waste as adding biosorbent. The adsorption process best followed the pseudo-second-order model, which is in agreement with chemical sorption being the rate-controlling step. The present study reveals that these cheap and easily available materials have the excellent adsorbing capability for copper removal from industrial effluents.
Acknowledgements
The authors would like to thank department of materials science and engineering at Shiraz University for providing laboratory facilities and Dr. M. A. Zare for providing atomic adsorption experiments.