Synthesis of Some New Bisindole Derivatives and Their Biological Activity ()
1. Introduction
Pyrrolidines are well known for their versatile pharmacological activities such as antimicrobial [1] [2] [3] [4] , antitumor [5] , anti HIV [6] , anticonvulsant [7] [8] , human melanocortin-4 receptor agonists [9] , etc. Moreover, indole nucleus is an important element of many natural and synthetic molecules that covers some of the relevant and recent achievement in the biological, chemical and pharmaceutical activity of important indole derivatives [10] . In view of these observations, the intention is directed to synthesize some new bisindole derivatives of expected biological interest.
2. Experimental
2.1. General
All melting points are uncorrected. IR spectra were recorded (KBr) with a Perkin- Elmer 1430 spectrophotometer. 1H NMR spectra were obtained on Varian EM 399.65 MHz equipment. MS spectra were recorded with a Jeol the MS route JMS-600 H. Substituted 1,4-diaza-1,3-butadienes (1a-f) were prepared according to known procedures by condensation of glyoxal and aromatic amines.
2.2. 6,13-Disubstituted 6,6a,13,13a-Tetrahydro-(5aH,5bH,12aH, 12bH)-Bisbenzo[f]Benzo-[5,6]Indolo[3,2-b]Indole-5,7,12,14- Tetrone (3a-f): General Procedure
A mixture of substituted 1,4-diaza-1,3-butadienes (1, 1 mmole) and 1,4-naphthoquinone (2, 2 mmoles) was refluxed in absolute ethanol or benzene for 8 - 12 hrs. The solution was filtered while hot. The mother liquor was concentrated and cooled. The highly colored (brown violet) products were filtered off and crystallized from ethanol. Yield 40% - 55%.
2.2.1. 6,13-Diphenyl-6,6a,13,13a-Tetrahydro-(5aH,5bH,12aH, 12bH)-Bisbenzo[f]Benzo-[5,6]Indolo[3,2-b]Indole-5,7,12,14-Tetrone (3a)
Adduct (3a), R = -C6H5, is obtained from 1,4-diphenyl-1,4-diazabuta-1,3-diene (1a) and (2) in 50% yield, m.p. 184˚C. Anal. calcd. for C34H24N2O4 (524.58): C, 77.85; H, 4.61; N, 5.34. Found: C, 77.58; H, 4.79; N, 5.42. IR (cm−1), υ: 1669 (C=O), 1260, 1445 (C-N). MS m/z (%): [M+-PhCO-O-H] 402 (0.1), common fragment ion [M+- 2 C6H5 - 2O] 337.8 (10.7).
2.2.2. 6,13-Di-p-Tolyl-6,6a,13,13a-Tetrahydro-(5aH,5bH,12aH, 12bH)- Bisbenzo[f]Benzo-[5,6]Indolo[3,2-b]Indole-5,7,12,14-Tetrone (3b)
Adduct (3b), R = -C6H4-p-CH3, is obtained from 1,4-di-p-tolyl-1,4-diaza-1,3-butadiene (1b) and (2) in 40% yield, m.p. 188˚C - 190˚C. Anal. calcd. for C36H28N2O4 (552.64): C, 78.24; H, 5.11; N, 5.07. Found: C, 78.37; H, 5.26; N, 5.14. IR (cm−1), υ: 1670 (C=O), 1246, 1508 (C-N). 1H NMR (CDCl3), d: 2.44 (s, 6H, 2-CH3), 3.7 (2H, 2CH-N), 6.3 (2H, 2-CH-CO), 6.8 - 8.2 (m, 2H, 2-CO-CH-N-, 16 H, Ar-H). MS m/z (%): [M+-2O-C2] 466.6 (0.2), common fragment ion ]M+- 2 C6H4-p-CH3 - 2 O] 337.79 (11.4).
2.2.3. 6,13-Di-p-Methoxyphenyl-6,6a,13,13a-Tetrahydro-(5aH,5bH, 12aH,12bH)-Bisbenzo-[f]Benzo[5,6]Indolo[3,2-b]Indole-5,7,12,14- Tetrone (3c)
Adduct (3c), R= -C6H4-p-OCH3, is obtained from 1,4-di-p-methoxyphenyl-1,4-diaza- 1,3-butadiene (1c) and (2) in 42% yield, m.p. 140˚C. Anal. calcd. for C36H28N2O6 (584.63): C, 73.96; H, 4.83; N, 4.79. Found: C, 73.49; H, 4.68; N, 4.90. IR (cm−1), υ: 1670 (C=O), 1249, 1508 (C-N). MS m/z (%): [M+-Ph-C2] 483.45 (0.2), common fragment ion [M+ - 2 C6H4-p-OCH3 - 2 O] 337.9 (0.9).
2.2.4. 6,13-Di-p-Hydroxyphenyl-6,6a,13,13a-Tetrahydro-(5aH,5bH, 12aH,12bH)-Bisbenzo-[f]Benzo[5,6]Indolo[3,2-b]Indole-5,7,12,14- Tetrone (3d)
Adduct (3d), R= -C6H4-p-OH, is obtained from 1,4-di-p-hydroxyphenyl-1,4-diaza-1,3- butadiene (1d) and (2) in 45% yield, m.p. 240˚C. Anal. calcd. for C34H24N2O6 (556.58): C, 73.37; H, 4.35; N, 5.03. Found: C, 73.19; H, 4.22; N, 4.90. IR (cm−1), υ: 3300 (OH), 1671 (C=O), 1267, 1435 (C-N). 1H NMR (CDCl3): d 3.7 (s, 2H, N-CH-CH-N), 6.2 (s, 2H, 2-CH-CO), 6.88 - 8.2 (m, 2H, 2-CO-CH-N, 16H, Ar-H), 8.6 (s, 2H, 2-OH). MS m/z (%): [M+-2C6H4-OH-2O] 338.2 (3.8), common fragment ion [M+ - 2 C6H4OH - 2 O] 337.92 (6.5).
2.2.5. 6,13-Di-p-Chlorophenyl-6,6a,13,13a-Tetrahydro-(5aH,5bH, 12aH,12bH)-Bisbenzo-[f]Benzo[5,6]Indolo[3,2-b]Indole-5,7,12,14- Tetrone (3e)
Adduct (3e), R= -C6H4-p-Cl, is obtained from 1,4-di-p-chlorophenyl-1,4-diaza-1,3- butadiene (1e) and (2) in 50% yield, m.p. 205˚C. Anal. calcd. for C14H10N2Cl (593.47): C, 68.81; H, 3.74; Cl, 11.95; N, 4.72. Found: C, 68.69; H, 3.61; Cl, 11.68; N, 4.91. IR (cm−1), υ: 1670 (C=O), 1299, 1406 (C-N). MS m/z (%): [M+-PhCO-H] 487 (1.3), common fragment ion [M+ - C6H4-p-Cl - 2 O] 337.9 (100).
2.2.6. 6,13-Di-α-Naphthyl-6,6a,13,13a-Tetrahydro-(5aH,5bH,12aH, 12bH)- Bisbenzo-[f]Benzo[5,6]Indolo[3,2-b]Indole-5,7,12,14-Tetrone (3f)
Adduct (3f), R= -C10H7, is obtained from 1,4-di-α-naphthyl-1,4-diaza-1,3-butadiene (1f) and (2) in 38% yield, m.p. 215˚C. Anal. calcd. for C42H28N2O4 (624.7): C, 80.75; H, 4.52; N, 4.48. Found: C, 80.29; H, 4.79; N, 4.61. IR (cm−1), υ: 1670 (C=O), 1269, 1455 (C-N). MS m/z (%): [M+-2C10H7-C2H2-2H] 342 (0.1), common fragment ion [M+ - 2 C10H7 - 2 O] 337.9 (20.9).
2.3. Antimicrobial Activity*
Antibacterial screening of compounds (3a-f) were determined by Nutrient agar well diffusion method. The tested compounds (3a-f) were dissolved in ethylene glycol to give 2% concentration. Antibacterial activity was determined according to the method reported by Bauer et al. [11] using 3 mm filter paper discs (Watmann No. 2) loaded with 10 μL of the solution under investigation (2.0%). The discs were placed on the surface of the bacterial culture, which were incubated at 30˚C. The diameter of the inhibition zone around each disc was measured (cf. Table 1).
3. Results and Discussion
Wagner [12] found that heating one mole of benzalazine with 2 moles of maleic anhydride to 100˚C in dry benzene for several hours gave addition compound through simultaneous 1,3- & 2,4-additoin of 2 moles of maleic anhydride to benzalazine, which was designated as criss-cross addition [13] . Moreover, aromatic 1,4-disubstituted 1,4-diazabuta-1,3-dienes with thiocyanates in glacial acetic acid via criss-cross cycloaddition produced the corresponding perhydroimidazo[4,5-d]imidazole-2,5-dithiones [14] . Similarly as continuation of my previous work [15] [16] [17] [18] [19] , for the
![]()
Table 1. Bactericidal activity expressed as inhibition zone in mm.
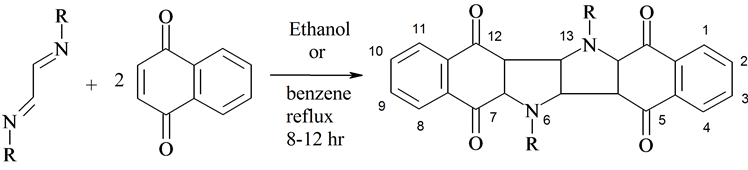
(1a-f) (2) (3a-f)
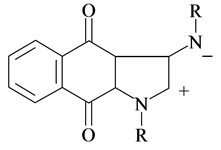
(4)
Scheme. R = -C6H5 (1a, 3a); -C6H4-p-CH3 (1b, 3b); -C6H4-p-OCH3 (1c, 3c); -C6H4-p-OH (1d, 3d); -C6H4-p-Cl (1e, 3e); -C10H7 (1f, 3f)
following criss-cross cycoaddition (Scheme), substituted 1,4-diaza-1,3-butadienes (1a- f) were allowed to react with 2 equivalents of 1,4-naphthoquinone (2), one pot reaction in presence of ethanol or benzene and afforded bisindoletetrone derivatives (3a-f). The structure of the synthesized compounds was assigned by elemental and spectral analysis.
Huisgen [20] suggested that criss-cross reaction can be represented by series of two [3+2] cycloaddition steps.
Compound (4) was postulated as a key intermediate. The compounds produced satisfactory results for elemental and spectral analysis.
As for their antimicrobial properties, some compounds exhibited strong activity such as (3c) (against Rhodopseduomonas fp. & E. coli [HD701]), (3d) (against Micrococcus luteus & E. coli), and (3e) (against Bacillus cereus). Others showed remarkable activity such as (3a) (against E. coli [HD701]), (3b) (against Micrococcus luteus & Rhodopseudomonas fp.) while other compounds showed week or no potency (3f).
4. Conclusion
The title compounds are synthesized successfully through simple novel route and one pot reaction from substituted 1,4-diaza-1,3-butadienes and 1,4-naphthoquinone. These compounds also exhibited strongly to remarkable bactericidal activity against some tested bacteria.
NOTES
*Done by Dr. Amal W. Danial, Botany & Microbiology Department, Faculty of science, Assiut University, Assiut 71526, Egypt.