Photo-Degradation of Reactive Yellow 14 Dye (A Textile Dye) Employing ZnO as Photocatalyst ()
1. Introduction
Reactive dyes are widely utilized of cellulose fibers in the textile industry in all the worlds that attitude has important properties, such as having many varieties of brilliant shades colours, water-soluble, cheaper in price, forming a chemical covalent bonds with the hydroxyl group of cellulose fibers, anionic in nature and very good colour fastness to washing. In sprit of having a high water-soluble, that is beyond to the natural of the chromophoric groups (like azo, metallized azo, phthalocyanine, anthraquinone, formazane and oxazine group) and the bridging groups (like ether or ester linkage as a covalent chemical bond) that linkage occurs between the dye and the fibers [1] [2] [3] [4] .
Reactive dyes are primarily used for dyeing and printing of cotton fibers. That will produce a high amount of wastewater. In the last decades, a large number of research groups worldwide focused on the techniques that were useful for the removal of dye from wastewater.
Roglić and coworkers [5] studied the electrochemical oxidation of groups from reactive textile dyes by carrying out in an electrochemical cell containing standard membrane industry; the highest degradation of study dyes was achieved at 12 V. The COD values for all dyes dropped at less 30 ppm O2 after 60 min, except reactive black 5 dyes who are reduced the COD with and without membrane at 57.95% and 35.28% respectively.
Tomczak and Tosik [6] found that the sorption capacity for reactive blue 81 onto a rye straw as a cheap plant sorbent was less favorable than it was for direct orange 26 after applied Freundlich, Langmuir, Redlich-Peterson and Radke-Prausntiz isotherm.
Sahasrabudhe and Pathade [7] isolated organism Enterococcus faecalis strain YZ 66 as a useful tool to remove the reactive yellow 145 dye from the wastewater, and the decoluorization of dye occurred at pH 5 and 37˚C. In other studied, they selected the reactive orange 16 for biotransformation studied by the same organism (Enterococcus faecalis strain YZ 66). They found that the decolourization of this dye was performed at 80 min with 77.73% under the optimum conditions [8] .
Emara and coworkers [9] degraded the reactive red 84 dye during 4 min only at 25 ppm of it, pH 1 by using 3.91 × 10−5 M of H2O2 and UV lamp emitted at 254 nm. Agustina and Ang [10] focused on the decolourization and the mineralization of reactive blue 4 and reactive red 2 by fenton reagent [Fe2+]/[H2O2] as one of the advance oxidation processes. The maximum degradation was found to be 99.8% at 60 min, pH 3, the ratio of [Fe2+]/[H2O2] equal 1:20 and TOC degradation of 39.8% - 42.5%. Racyte and coworker [11] changed the pH of the raw textile wastewater that contained of reactive yellow 84 and reactive red 141. The initial pH of the solution is 11.4, but when acidification, the solution to pH 7 leads to increase the rate constant to higher 10 times than it is at pH 11.4. Then the rate constant increased to 25 times with change the pH of solution from 11.4 to 3 and the time of decolourization was reduced from 5 h to 2 h at pH 3.
Alizadeh and coworkers [12] found to be that the optimum pH for favorable adsorption of reactive green 19, reactive yellow 14 and reactive orange 16 dyes on the peanut shell powder was 2 at 90 min, and the removal percentage was equal to 96.3%, 98.25 and 98.4% respectively. The isotherm data fitted the Freundlich model.
The target of present work is to investigate the photocatalytic decolorization of the reactive yellow 14 dye (Figure 1) in commercial ZnO suspension solution by light source type UV (A) at different conditions.
2. Materials and Methods
Reactive yellow 14 dye (C20H19ClN4Na2O11S3) is commonly employed in textile industries, it was supplied by the Hilla textile factory. Zinc oxide (99.5% purity, mean crystallite sizes is 44.116 nm) was supplied by Fluka. All the chemicals employed in photocatalytic experiment were of the highest purity available and of analytical grade. The
![]()
Figure 1. The structure formula of reactive yellow 14 dye.
required amount of the ZnO used as a catalyst was suspended in 100 mL of reactive yellow 14 dye employing a Labtech magnetic stirrer. At a regular intervals; about 3 mL of the mixture was collected and centrifuged (4000 rpm, 15 minutes) in a Hettich centrifuge. Then, the filtration solution was carefully transformed to a new plastic test tube by a syringe after centrifuged again (4000 rpm, 10 minutes). This centrifugation was useful to remove the fine ZnO particles. At predetermined times of irradiation; the concentrations of residual reactive yellow 14 dye were altered and determined through the UV-visible spectro-photometer type Labomad, USA at 415 nm. The chemical actinometric solution [13] was employed to determine the light intensity of the irradiation source in the photo reactor (Philips 250 W, high pressure mercury lamp, Germany), and the value found to be 2.995 × 10−8 Ens. s−1. A general diagram of the experimental set-up is shown in Scheme 1.
3. Results and Discussion
3.1. Photocatalytic Kinetic Analysis
The experimental results indicated to nodecolourization of reactive yellow 14 dye in the existence of ZnO and without light (dark reaction). On the other hand, no decolourization of this dye was observed with irradiated reaction in the absence of ZnO (photolysis reaction). The results proved that the decolourization of the dye was dependent on the presence of UV light and a catalyst. That regarded as fundamental for the effective destruction of reactive yellow 14 dye.
According to the Langmuir-Hinshelwood (L-H) model, at assumption the initial dye concentration is low; the rate of reaction follows pseudo first-order kinetics, so the apparent rate constant (kapp) expression was calculated by the following equations [14] :
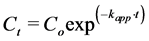 (1)
(1)
where: Co is an initial concentration of reactive yellow 14 dye at time of irradiation = 0 min. Ct is a concentration of the same dye at time t of irradiation.
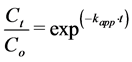 (2)
(2)
or
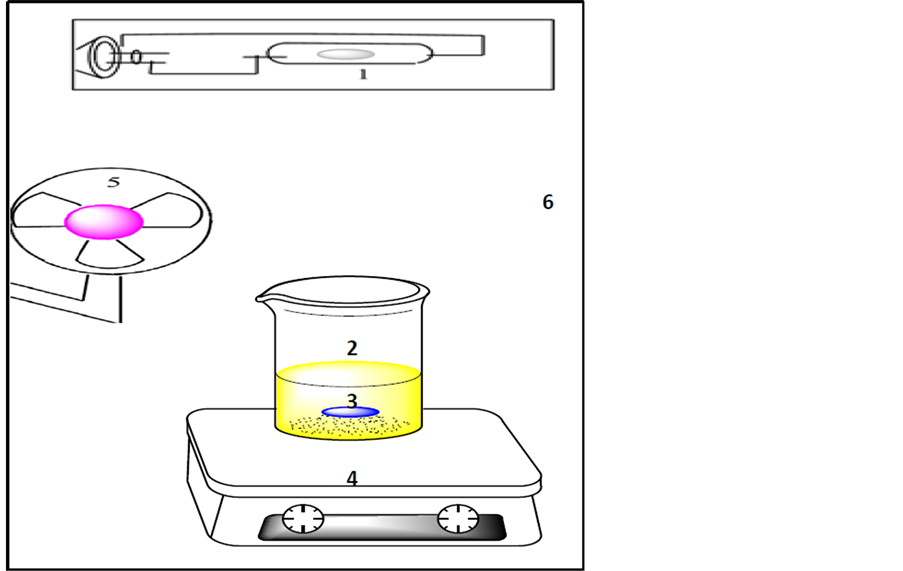
Scheme 1. Schematic Diagram of Experimental Set-up. Where: High pressure mercury lamp (250 W) (1), 400 cm3 Pyrex glass beaker (2), Teflon bar (3), magnetic stirrer (4), fan (5), and wooden box (6).
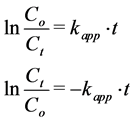 (3)
(3)
The Langmuir-Hinshelwood (L-H) kinetics model has four possible [15] [16] :
1) The reaction occurs between two adsorbed substances (Dye(ads.) and •OH(ads.)).
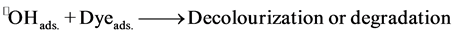 (4)
(4)
2) A nonbound radical (radical in solution) reacts with an adsorbed dye molecular (Dye(ads.) and •OH(sol.)).
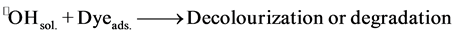 (5)
(5)
3) The reaction occurs between a radical linked to the surface and substance molecular in solution (Dye(sol.) and •OH(ads.)).
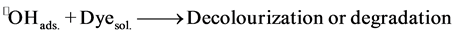 (6)
(6)
4) The reaction obtains between two free species in solution (Dye(sol.) and •OH(sol.)).
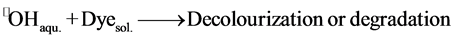 (7)
(7)
In all cases, the express of reaction rate equation is similar.
3.2. Effect of Dye Concentration
According to Figure 2, the rate constant of decolourization of reactive yellow 14 dye was inversely proportional with increasing the initial dye concentrations at ranged (50 - 100) ppm, that due to increase the dye concentration but the catalyst amount is kept constant, hence the active sites is fewer. Moreover, this behavior isinterpreted to decline the reached optical density of light in the solution [12] [17] and according to the Equations (4) - (7) for Langmuir-Hinshelwood (L-H) kinetics model possible, the increased of the dye concentration compared with the amount of •OH that source it mostly is water, then O2 and OH of catalyst, that leads to depress of the rate of decolourization.
![]()
Figure 2. Pseudo-first order rate constant at varying dye concentration. Conductions: semiconductor dosage 350 mg/100mL, pH = 6.75, temperature 311.15 K, UV light intensity 2.995 × 10−8 ensien∙s−1.
3.3. Effect of ZnO Dose
In order to shun the unnecessary excess of catalyst and to sponsor a wholly absorption of light without loss, the dose of catalyst must be determined.
The effect of ZnO dosage is depicted in Figure 3. The results show that the measured rate constant of reaction were found to be increased with increased the catalyst dosage from 100 to 350 mg/100mL, this behavior reflects the increment of the numbers of the active sites of catalyst, which in turn increases the rate of radical formation [17] [18] .
![]()
Figure 3. Pseudo-first order rate constant at varying semiconductor dosages. Conductions: dye conc. 50 ppm, pH = 6.75, temperature 311.15 K, UV light intensity 2.995 × 10−8 ensien∙s−1.
The maximum decolourization rate level is observed with 350 mg/100mL that regarded as the optimum catalyst ZnO dosage [19] . From other side, at a high dose of catalyst, the rate of reaction and the penetration of light depresses that attitude to increase the turbidity of solution and light scattering this called screening effect [13] [20] [21] .
3.4. Effect of pH
The initial pH is considered one of the most benefit parameters that affects not only on the surface charge of catalyst, but also the characteristics of textile dye, and generation of hydroxyl radicals, that enhancements the photode colourization of the organic pollutants such as a textile dye in presence of photocatalyst [17] .
From Figure 4, the experimental results demonstrated that the initial pH of reactive yellow 14 dye (6.75) with 50 ppm was the most active in photo decolourization process. The minimum rate constant was recorded at initial pH 4 and 11, thereby; there are two interpretations for understanding the effect of pH on the rate of reaction: 1) surface state of catalyst & 2) ionization state of ionisable organic molecules [22] .
The first interpretation can be performed in acidic and basic medium. At acidic pH, ZnO undergoes photo-corrosion through self-oxidation according to the following equations [18] [23] :
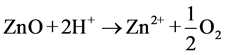 (acidic pH) (8)
(acidic pH) (8)
or
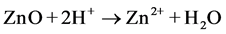 (acidic pH) (9)
(acidic pH) (9)
While, at basic pH, the ZnO can undergo dissolution, so the photocatalytic activity of ZnO depresses [18] .
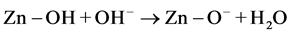 (basic pH) (10)
(basic pH) (10)
or
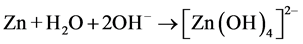 (basic pH) (11)
(basic pH) (11)
![]()
Figure 4. Pseudo-first order rate constant at varying initial pH. Conductions: dye conc. 50 ppm, semiconductor dosage 350 mg/100mL, temperature 311.15 K, UV light intensity 2.995 × 10−8 ensien∙s−1.
3.5. Effect of Temperature
At different temperatures in the range 278.15 - 311.15 K, Figure 5 and Figure 6 obtain that the efficiency of decolourization for reactive yellow 14 dye is a linear relationship, that fitting the graph of the Arrhenius Equation (12) and of Eyring Equation (13). Equation (12) was used to calculate the apparent activation energy [24] .
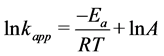 (12)
(12)
where: kapp is apparent rate constant; Ea is apparent activation energy; R is gas constant; T is temperature of reaction and A is a frequency constant.
The decolourization of dye was increased with increasing temperature. The apparent activation energy on ZnO surface for this dye was calculated and found to be 27.244 kJ∙mol−1. From the other hand, the thermodynamic parameters enthalpy of activation ΔH#, entropy of activation ΔS# were evaluated by depending on the plot of Eyring Equation (13). The free energy activation ΔG# is calculated using Gibbs Equation (14) [24] .
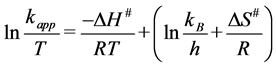 (13)
(13)
![]()
Figure 5. Pseudo-first order rate constant at varying temperature. Conductions: dye conc. 50 ppm, semiconductor dosage 350 mg/100mL, pH = 6.75, temperature 278.15 - 311.15 K, UV light intensity 2.295 × 10−8 ensien∙s−1.
![]()
Figure 6. Eyring plot of (ln(Kapp/T)) vs.1/T. Conductions: dye conc. 50 ppm, semiconductor dosage 350 mg/100mL, pH = 6.75, 278.15 - 311.15 K, UV light intensity 2.295 × 10−8 ensien∙s−1.
where: kB is a Boltzmann’s constant; h is a Plank’s constant; R is a gas constant and T is the temperature of reaction.
![]() (14)
(14)
The fitted results of the apparent activation energy and the other thermodynamics functions are shown in Table 1.
![]()
Table 1. The activation kinetic and thermodynamic parameters of the decolourization of reactive yellow 14 dye.
![]()
Scheme 2. Suggested mechanism of photo degradation of reactive yellow 14 dye.
According to the results in Table 1, the high positive ΔH# and ![]() proved that the transition state between the dye molecules and hydroxyl radicals (as reaction intermediate) is highly solvated structure, that also supported the small negative value of ΔS# and indicates that the complex formed is less random than reactants [17] [25] [26] . The decolourization process of this dye was endothermic reaction and no spontaneously (ΔH# and
proved that the transition state between the dye molecules and hydroxyl radicals (as reaction intermediate) is highly solvated structure, that also supported the small negative value of ΔS# and indicates that the complex formed is less random than reactants [17] [25] [26] . The decolourization process of this dye was endothermic reaction and no spontaneously (ΔH# and ![]() are positive).
are positive).
3.6. Suggested Mechanism
When the photon of UV light focuses on the colloid solution of direct orange dye with ZnO, series of chain oxidative-reductive reactions occur. The photoelectron and photohole (e− − h+)exciton were generated. Photoelectron can be reacted with O2 of airenvironment and produced![]() . Moreover,
. Moreover, ![]() reacts with H+ that beyond to hydrolysis of water to formed •O2H and •OH. The •OH generates from −OH of water or ZnO surface react with the photohole [26] [27] [28] . From other word, the photohole and photoelectron input in serious of reactions to degradation the dye by the more active species (
reacts with H+ that beyond to hydrolysis of water to formed •O2H and •OH. The •OH generates from −OH of water or ZnO surface react with the photohole [26] [27] [28] . From other word, the photohole and photoelectron input in serious of reactions to degradation the dye by the more active species (![]() , •O2H and •OH) as follow in Scheme 2.
, •O2H and •OH) as follow in Scheme 2.
4. Conclusions
Photo decolourization of reactive yellow 14 dye was kinetically studied and proved that the photoreaction obeyed the pseudo first order. Moreover, it’s found that the method of decolourization is dependent on parameters such as dye concentration, catalyst dosages, initial pH of solution and temperature.
The optimum concentration of dye was 50 ppm and the best dosage of ZnO found at 3.5 g/L, and the maximum value of pH was reached to 6.75. The activation energy for decolourization of dye was calculated and equal to 27.244 kJ∙mol−1 by using Arrhenius equation. Further the thermodynamics functions were calculated by depending on Eyring equation. The photoreaction process was observed to be endothermic reaction non-spontaneous and less randomness.