Received 13 December 2015; accepted 1 March 2016; published 4 March 2016

1. Introduction
One of the most common procedures for the hydrogenation of alkenes in a chemistry laboratory is the hydrogenation over palladium catalysts such as over palladium on carbon (Pd/C). Because of the danger of working with H2 in our laboratory, we looked for a reaction system that would generate H2 in situ. Recently, A. T. Russo et al. [1] [2] have published reaction conditions (NaBH4, CH3CO2H, Pd/C) that would achieve this. We could utilize this system, e.g., in the hydrogenation of 1 to 2 (Scheme 1), where 2 is a precursor to quinines 3 linked to a carrier. At the time, we exchanged the published solvent of the reaction, toluene, to the more easily removable benzene.
One of the common protective groups for the alcohol (OH) function and for the carboxylic acid (CO2H) function is the benzyl moiety in form of an O-benzyl ether (OCH2Ph) and O-benzyl ester (CO2CH2Ph) [3] . Often, both can be removed by hydrogenolysis when using a palladium on carbon (Pd/C) catalyst [4] . Also, an N-func- tion, such as in an amide, can be protected with a benzyl group, where the group is subsequently removed by Pd-catalysed hydrogenation. Under the conditions of the reductive debenzylation, double bonds can also be
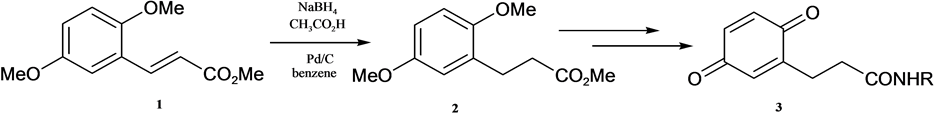
Scheme 1. Olefin hydrogenation with NaBH4, CH3CO2H, Pd/C.
hydrogenated, of course. If the actual desired transformation, however, is to be the hydrogenation of a double bond in the substrate, then one risks losing the benzyl functions as protective groups in the molecule at the same time. Oftentimes, the hydrogenation reaction is not chemoselective, but coincides with the reduction of nitro groups, azide functions, dehalogenations and also with O- and N-debenzylations of O-benzyl ethers and esters, N-benzyl amines and amides. In recent times, more chemoselective catalysts have been developed, mainly based on platinum group metals. These catalysts include polymer-imprinted platinum [5] , ZnX2-Pd/C and Pt/C systems [6] and platinum sulfides [7] , and specifically prepared Pd-catalysts [8] . Also, the addition of amines [9] [10] or diphenyl sulfide [11] to Pd/C or Pt/C has been found to make the catalysts more chemoselective, where the hydrogenation of alkenes is not accompanied by all of the side reactions mentioned above. With all of the above catalysts available, it is still of importance to develop new chemoselective hydrogenation systems, where the catalysts can be simply prepared.
In the following, the authors show that the reaction system NaBH4, CH3CO2H, Pd/C can be used for the hydrogenation of alkenes without the loss of O-benzyl or N-benzyl groups, so that benzyl ethers, benzyl esters and N-benzyl amides are not converted concurrently to alcohols, acids, and amides, respectively.
2. Experimental
2.1. Chemicals and Instruments
Melting points were measured on a Stuart SMP 10 melting point apparatus and are uncorrected. Infrared spectra were measured with a Thermo/Nicolet Nexus 470 FT-IR ESP Spectrometer. 1H and 13C NMR spectra were recorded with a Varian 400 NMR (1H at 395.7 MHz, 13C at 100.5 MHz) and a Varian 200 MHz NMR spectrometer (1H at 200.0 MHz, 13C at 50.3 MHz). The chemical shifts are relative to TMS (solvent CDCl3, unless otherwise noted). Mass spectra were measured with a JMS-01-SG-2 spectrometer. CHN-analysis was performed on a LECO TruSpec Micro instrument. Column chromatography was carried out on silica gel (60 A, 230 - 400 mesh, Sigma-Aldrich).
5w% Palladium on carbon (Aldrich, 205680) was used in all experiments. NaBH4 and acetic acid were acquired commercially. Benzene, toluene and THF were used without prior purification. Benzyl esters 12, 16, 18, 36, and 40 were prepared from the corresponding acids (benzyl alcohol, PPh3, BrCCl3, CH2Cl2) following a known procedure [12] . Methyl ester 14 was prepared by Wittig olefination from 3-benzyloxy-4-methoxyben- zaldehyde and methoxycarbonylmethylidenetriphenylphosphorane in a minimal amount of CHCl3. Also, N-benzyl amides 27, 29, 31 and 33 were synthesized from the corresponding acids (benzylamine, PPh3, BrCCl3, CH2Cl2) [12] . Substituted dibenzyl ethers 38 and 43 were obtained by Wilkinson-type etherification (ArCH2OH, benzyl chloride, KOH, DMSO) as was 2-benzyloxycinnamaldehyde (24) (2-hydroxycinnamaldehyde, benzyl chloride, KOH, DMSO). 20 and 22 were prepared by Wittig olefination, starting from 2-benzyloxybenzaldehyde and benzoylmethylidenetriphenylphosphorane and from 2-benzyloxycinnamaldehyde (24) and toluoylmethylidenetriphenylphosphorane.
Caution: In the presence of dry palladium on carbon, hydrogen enflames upon contact with air. Therefore, it is advisable to purge the reaction flasks with an inert gas before use in the described hydrogenation. Also, where filtrating the reaction mixture directly, especially when using a paper filter, it must be noted that the filter cake upon drying can enflame due to the fact that unreacted sodium borohydride slowly hydrolyses with air moisture, thereby releasing hydrogen. Therefore, after diligent washing with chloroform, the filter and filter cake should be immersed in water.
2.2. General Procedure for the Hydrogenation of Cinnamates.-Methyl 3-[2-Benzyloxyphenyl]Propionate (13) [13]
To a solution of methyl o-benzyloxycinnamate (6, 188 mg, 0.70 mmol) in benzene (10 mL) is given Pd/C (70 mg, 5 wt%) and acetic acid (AcOH, 100 mg). Thereafter, is added portionwise NaBH4 (128 mg, 3.38 mmol). After 3 h at rt, further AcOH (50 mg) and NaBH4 (60 mg, 1.58 mmol) are added successively, and the resulting mixture is stirred at rt for 12 h. Thereafter, half conc. aq. HCl is added dropwise until there is no futher gas evolution. H2O (30 mL) is added and the mixture is extracted with CH2Cl2 (3 × 20 mL). The combined organic phase is dried over anhydrous MgSO4, concentrated in vacuo and the residue is subjected to column chromatography on silica gel (CH2Cl2) to give 13 (175 mg, 93%) as a colorless oil; νmax (neat/cm−1) 3064, 3033, 2950, 1736, 1601, 1588, 1493, 1453, 1436, 1381, 1290, 1241, 1193, 1162, 1025, 752; δH (400 MHz, CDCl3) 2.65 (2H, t, 3J = 7.6 Hz), 3.01 (2H, t, 3J = 7.6 Hz), 3.64 (3H, s, OCH3), 5.09 (2H, s, OCH2), 6.87 - 6.92 (2H, m), 7.16 - 7.46 (8H, m); δC (100.5 MHz, CDCl3) 26.2 (CH2), 34.0 (CH2), 51.5 (OCH3), 69.7 (OCH2), 111.5 (CH), 120.7 (CH), 127.0 (2C, CH), 127.6 (CH), 127.8 (CH), 128.6 (2C, CH), 129.1 (Cquat), 130.1 (CH), 137.2 (Cquat), 156.5 (Cquat), 173.8 (Cquat, CO); MS (EI, 70 eV) m/z (%) 270 (M+, 85).
2.3. General Procedure for the Hydrogenation of Cinnamides.-N-Benzyl 3-Phenylpropionamide (28) [14]
To a mixture of N-benzyl cinnamide (335 mg, 1.41 mmol) and Pd/C (70 mg, 5 w%) in toluene (8 mL) was added acetic acid (210 mg) and subsequently NaBH4 (185 mg). After the mixture was stirred for 14 h, it was filtered, and the filter cake was washed with CHCl3 (3 × 15 mL). The combined organic phase was concentrated in vacuo, and the residue was subjected to column chromatography on silica gel (ether/CHCl3/hexane 2:2:1) to give 28 (315 mg, 95%) as a colorless solid, mp. 90˚C - 93˚C; νmax (KBr/cm−1) 3292 (s, NH), 3061, 3026, 2924, 1639, 1543, 1495, 1453, 1227, 1029, 741, 694; δH (400 MHz, CDCl3) 2.51 (2H, t, 3J = 7.6 Hz), 2.99 (2H, t, 3J = 7.6 Hz), 4.38 (2H, d, 3J = 5.6 Hz), 5.66 (1H, bs, NH), 7.12 - 7.29 (10H, m); δC (100.5 MHz, CDCl3) 31.7 (CH2), 38.5 (CH2), 43.6 (CH2), 126.3 (CH), 127.5 (CH), 127.7 (2C, CH), 128.4 (2C, CH), 128.6 (2C, CH), 128.7 (2C, CH), 138.1 (Cquat), 140.7 (Cquat), 171.9 (Cquat, CO).
2.4. Reduction of Nitro-Containing Compounds―Variant A: Anthranilic Acid Benzyl Ester (41) [15]
To a mixture of benzyl 2-nitrobenzoate (40, 361 mg, 1.4 mmol), Pd/C (100 mg, 5w%) and AcOH (210 mg) in benzene (10 mL) is slowly added NaBH4 (185 mg, 4.87 mmol), and the resulting reaction mixture is stirred at rt for 14 h. Thereafter, the mixture is filtrated and the filter cake is washed with CHCl3 (2 × 20 mL). Column chromatography on silica gel (ether/CH2Cl2 1:10 → ethyl acetate/hexane 1:1) gave 41 (225 mg, 71%) as a pale solid; mp. 75˚C (Lit. 76˚C - 77˚C [15] ); νmax (KBr/cm−1) 3033, 2950, 1693, 1615, 1487, 1455, 1378, 1291, 1243, 1161; δH (400 MHz, CDCl3) 5.34 (2H, s OCH2), 6.62 - 6.68 (2H, m), 7.24 - 7.41 (4H, m), 7.44 (2H, d, 3J = 8.8 Hz), 7.93 (1H, d, 3J = 8.0 Hz); δH (400 MHz, CDCl3) 66.0 (OCH2), 110.7 (Cquat), 116.4 (CH), 116.7 (CH), 128.0 (2C, CH), 128.1 (CH), 128.6 (2C, CH), 131.3 (CH), 134.2 (CH), 136.3 (Cquat), 150.5 (Cquat), 167.9 (Cquat, CO) and 42 (38 mg, 20%).
2.5. Reduction of Nitro-Containing Compounds―Variant B: Anthranilic Acid (42) [16]
To a mixture of benzyl 2-nitrobenzoate (40, 361 mg, 1.4 mmol), Pd/C (100 mg, 5 w%) and AcOH (210 mg) in benzene (10 mL) is slowly added NaBH4 (185 mg, 4.87 mmol), and the resulting reaction mixture is stirred at rt for 14 h. Then, additional AcOH (105 mg) and NaBH4 (100 mg, 2.63 mmol) were added, and the reaction was stirred at rt for an additional 10h. Thereafter, half-conc. aqHCl is added dropwise. Subsequently, water (25 mL) is added, and the mixture is extracted with ethyl acetate (3 × 20 mL). The combined organic phase was dried over anhydrous MgSO4 and concentrated in vacuo. The residue was subjected to column chromatography on silica gel (ethyl acetate-hexane 1:1) to give anthranilic acid (42, 163 mg, 85%) as a beige-colored solid, mp. 144˚C - 146˚C (Lit. 146˚C - 147˚C [16] ); νmax (KBr/cm−1) 3472 (NH), 3373 (NH), 3040 - 2350 (bs, OH), 1672, 1617, 1588, 1563, 1485, 1419, 1301, 1247, 1161, 916, 753, 659; δH (400 MHz, CDCl3) 6.65 - 6.69 (2H, m), 7.29 - 7.33 (1H, m), 7.92 (1H, dd, 3J = 8.4 Hz, 4J = 1.6 Hz); δH (400 MHz, CDCl3) 109.5 (Cquat), 116.5 (CH), 116.8 (CH), 132.1 (CH), 135.1 (CH), 151.1 (Cquat), 173.1 (Cquat, CO); MS (EI, 70 eV) m/z (%) 137 (M+, 64), 119 (100), 92 (81, M+-CHO2).
2.6. Spectral and Analytical Data
Ethyl 3-benzyloxy-4-methoxypropionate (15) [17] . -as a colorless oil; νmax (neat/cm−1) 1731 (CO), 1515; δH (400 MHz, CDCl3) 1.22 (3H, t, 3J = 7.2 Hz, CH3), 2.53 (2H, t, 3J = 7.2 Hz), 2.83 (2H, t, 3J = 7.2 Hz), 3.85 (3H, s, OCH3), 4.09 (2H, q, 3J = 7.2 Hz), 5.11 (OCH2), 6.73(5) (1H, dd, 3J = 8.4 Hz, 4J = 2.0 Hz), 6.74 (1H, d, 4J = 2.0 Hz), 6.81 (1H, d, 3J = 8.4 Hz), 7.27 - 7.44 (5H, m); δC (100.5 MHz, CDCl3) 14.2 (CH3), 30.5 (CH2), 36.1 (CH2), 56.1 (OCH3), 60.4 (OCH2), 71.0 (OCH2), 111.8 (CH), 114.4 (CH), 120.8 (CH), 127.3 (2C, CH), 127.8 (CH), 128.5 (2C, CH), 133.1 (Cquat), 137.1 (Cquat), 148.0 (Cquat), 148.1 (Cquat), 173.0 (Cquat, CO); MS (EI, 70 eV) m/z (%) 300 (M+, 31).
Benzyl 3-[2-benzyloxyphenyl]propionate (17) [18] .-colorless oil; νmax (neat/cm−1) 3064, 3033, 2933, 1735, 1601, 1588, 1491, 1450, 1382, 1232, 1110, 1009, 910, 853, 742, 696; δH (400 MHz, CDCl3) 2.71 (2H, t, 3J = 7.6 Hz), 3.05 (2H, t, 3J = 7.6 Hz), 5.09 (4H, s), 6.87 - 6.89 (2H, m), 7.15 - 7.42 (12H, m); δC (100.5 MHz, CDCl3) 26.3 (CH2), 34.2 (CH2), 66.1 (OCH2), 69.7 (OCH2), 111.6 (CH), 120.8 (CH), 127.0 (2C, CH), 127.6 (CH), 127.7(5) (CH), 128.1 (2C, CH), 128.5 (3C, CH), 128.5(5) (2C, CH), 129.1 (Cquat), 130.1 (CH), 136.1 (Cquat), 137.2 (Cquat), 156.6 (Cquat), 173.2 (Cquat, CO); MS (EI, 70 eV) m/z (%) 346 (M+, 73).
Benzyl 3-[4-ethoxyphenyl]propionate (19). -colorless oil; νmax (KBr/cm−1) 3065, 3033, 2979, 2930 1736, 1612, 1512, 1454, 1383, 1297, 1242, 1150, 1116, 1048, 923, 825, 737, 698; δH (400 MHz, CDCl3) 1.40 (3H, t, 3J = 7.2 Hz), 2.64 (2H, t, 3J = 7.6 Hz), 2.90 (2H, t, 3J = 7.6 Hz), 3.99 (2H, q, 3J = 7.2 Hz, OCH2), 5.10 (2H, s, OCH2), 7.29 (2H, d, 3J = 7.6 Hz), 7.34 (2H, d, 3J = 7.6 Hz), 6.79 (2H, d, 3J = 8.8 Hz), 7.08 (2H, d, 3J = 8.8 Hz); δC (100.5 MHz, CDCl3) 14.9 (CH3), 30.1 (CH2), 36.2 (CH2), 63.4 (OCH2), 66.2 (OCH2), 114.4 (2C, CH), 128.2 (2C, CH), 128.5 (2C, CH), 129.2 (2C, CH), 132.3 (CH), 135.9 (Cquat), 138.9 (Cquat), 157.5 (Cquat), 172.8 (Cquat, CO); MS (EI, 70 eV) m/z (%) 284 (M+, 43).
2-(2’-Benzyloxyphenyl)ethyl phenylketone (21) [19] . -colorless oil; νmax (KBr/cm−1) 3063, 2929, 1682, 1598, 1495, 1450, 1240, 1111, 1021, 740; δH (400 MHz, CDCl3) 3.09 (2H, t, 3J = 7.2 Hz), 3.27 (2H, dt, 3J = 7.2 Hz, 4J = 1.2 Hz), 5.11 (2H, s, OCH2), 6.89 - 6.95 (2H, m), 7.17 - 7.25 (2H, m), 7.30 - 7.53 (8H, m), 7.90 (2H, d, 3J = 7.6 Hz); δC (100.5 MHz, CDCl3) 26.1 (CH2), 39.1 (CH2), 69.9 (OCH2), 111.6 (CH), 120.9 (CH), 127.3 (2C, CH), 127.5 (CH), 127.9 (CH), 128.1 (2C, CH), 128.5 (2C, CH), 128.6 (2C, CH), 129.8 (Cquat), 130.4 (CH), 132.8 (CH), 136.8 (Cquat), 137.2 (Cquat), 156.6 (Cquat), 200.1 (Cquat, CO); MS (EI, 70 eV) m/z (%) 316 (M+, 23).
1-Benzyloxy-2-[4-(4-methylbenzoyl)butyl]benzene (23). -colorless oil; νmax (KBr/cm−1) 3062, 3032, 2927, 2858, 1680, 1606, 1493, 1451, 1379, 1290, 1238, 1180, 1112, 1025, 752, 696; δH (400 MHz, CDCl3) 1.61 - 1.82 (4H, m), 2.40 (3H, s, CH3), 2.72 (2H, t, 3J = 7.2 Hz), 2.93 (2H, t, 3J = 7.2 Hz), 5.07 (2H, s, OCH2), 6.88 - 6.91 (2H, m), 7.13 - 7.44 (9H, m), 7.83 (2H, d, 3J = 8.0 Hz); δC (100.5 MHz, CDCl3) 21.6 (CH3), 24.3 (CH2), 29.6 (CH2), 30.1 (CH2), 38.3 (CH2), 69.8 (OCH2), 111.6 (CH), 120.7 (CH), 126.9 (CH), 127.1 (2C, CH), 127.7 (CH), 128.2 (2C, CH), 128.5 (2C, CH), 129.2 (2C, CH), 130.0 (CH), 131.1 (Cquat), 134.6 (Cquat), 137.5 (Cquat), 143.5 (Cquat), 156.5 (Cquat), 200.2 (Cquat, CO); MS (EI, 70 eV) m/z (%) 328 (M+, 17).
3-(2-Benzyloxyphenyl)propionaldehyde (25) [20] . -colorless oil; νmax (KBr/cm−1) 2929, 1722, 1600, 1493, 1452, 1382, 1238, 1118, 1019, 748; δH (400 MHz, CDCl3) 2.75 (2H, dt, 3J = 7.6 Hz, 4J = 1.6 Hz), 3.00 (2H, t, 3J = 7.6 Hz), 5.08 (2H, s), 6.87 - 6.92 (2H, m), 7.13 - 7.20 (2H, m), 7.30 - 7.43 (5H, m), 9.78 (1H, t, 3J = 1.6 Hz); δC (100.5 MHz, CDCl3) 23.5 (CH2), 43.9 (CH2), 69.8 (OCH2), 111.6 (CH), 120.8 (CH), 127.1 (2C, CH), 127.7 (CH), 127.9 (CH), 128.6 (2C, CH), 128.9 (Cquat), 130.1 (CH), 137.1 (Cquat), 156.5 (Cquat), 202.4 (CHO); MS (EI, 70 eV) m/z (%) 240 (M+, 13).
3-(2-Benzyloxyphenyl)propan-1-ol (26) [20] . -colorless oil; νmax (KBr/cm−1) 3351 (broad, OH), 3064, 3033, 2933, 2864, 1600, 1587, 1493, 1452, 1381, 1239, 1041, 910, 751, 696; δN (400 MHz, CDCl3) 1.86 (2H, tt, 3J = 7.2 Hz, 3J = 6.0 Hz, CH2), 2.78 (2H, t, 3J = 7.2 Hz, CH2), 3.60 (2H, t, 3J = 6.0 Hz, OCH2), 5.08 (2H, s, OCH2), 6.90 - 6.94 (2H, m), 7.15 - 7.20 (2H, m), 7.31 - 7.45 (5H, m); δC (100.5 MHz, CDCl3) 26.0 (CH2), 33.0 (CH2), 61.9 (OCH2), 70.1 (OCH2), 111.7 (CH), 121.0 (CH), 127.2 (CH), 127.3 (2C, CH), 128.0 (CH), 128.6 (2C, CH), 130.3 (CH), 130.4 (Cquat), 137.0 (Cquat), 156.6 (Cquat); MS (EI, 70 eV) m/z (%) 240 (M+, 25).
N-Benzyl 3-(2,5-dimethoxyphenyl)propionamide (30). -as a colorless solid, mp. 154˚C - 155˚C; νmax (KBr/ cm−1) 3311, 3063, 2948, 2839, 1638, 1593, 1541, 1474, 1255, 1161, 1113, 774; δH (400 MHz, CDCl3) 2.61 (2H, t, 3J = 7.2 Hz), 2.95 (2H, t, 3J = 7.2 Hz), 3.67 (2C, 2 OCH3), 6.42 (1H, s, NH), 6.48 (2H, d, 3J = 8.4 Hz), 7.13 (1H, dd, 3J = 8.4 Hz, 3J = 8.4 Hz), 7.15 - 7.20 (2H, m), 7.26 - 7.33 (3H, m); δC (100.5 MHz, CDCl3) 18.6 (CH2), 35.5 (CH2), 43.7 (CH2), 55.4 (2C, 2 OCH3), 103.6 (2C, CH), 116.5 (Cquat), 127.4 (2C, CH), 128.0 (2C, CH), 128.6 (2C, CH), 138.2 (Cquat), 157.9 (2C, Cquat), 173.1 (Cquat, CO); MS (FAB, 3-nitrobenyl alcohol) m/z (%) 300 (MH+, 15).
N-Benzyl 3-(3-methoxy-4-propoxyphenyl)propionamide (32). -as a colorless solid; mp. 127˚C; νmax (KBr/ cm−1) 3292 (NH), 3057, 3028, 2962, 2934, 2874, 1640, 1550, 1515, 1453, 1256, 1227, 1136, 1025, 803, 741, 697; δH (400 MHz, CDCl3) 1.02 (3H, t, 3J = 7.6 Hz, CH3), 1.85 (2H, qt, CH2, 3J = 7.6 Hz, 3J = 6.8 Hz), 2.49 (2H, t, 3J = 7.6 Hz, CH2), 2.92 (2H, t, 3J = 7.6 Hz, CH2), 3.80 (3H, s, OCH3), 3.92 (2H, t, 3J = 6.8 Hz, OCH2), 4.38 (2H, d, 3J = 1.2 Hz, CH2), 5.69 (1H, bs, NH), 6.69 (1H, dd, 3J = 8.0 Hz, 4J = 2.0 Hz), 6.71 (1H, d, 4J = 2.0 Hz), 6.76 (1H, d, 3J = 8.0 Hz), 7.24 - 7.31 (5H, m); δC (100.5 MHz, CDCl3) 10.5 (CH3), 22.5 (CH2), 31.4 (CH2), 38.8 (CH2), 43.6 (NCH2), 55.9 (OCH3), 70.5 (OCH2), 112.1 (CH), 113.0 (CH), 120.2 (CH), 127.5 (CH), 127.7 (2C, CH), 128.7 (2C, CH), 133.3 (Cquat), 138.1 (Cquat), 147.0 (Cquat), 149.3 (Cquat), 172.0 (Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 328 (MH+, 23).
N-Benzyl 3-benzyloxy-4-methoxyphenylpropionamide (34). -colorless solid, mp. 142˚C - 143˚C; νmax (KBr/ cm−1) 3301 (bs, NH), 2936, 1644 (C = O), 1514, 1252, 1154, 1133, 1110, 1049, 1016, 698; δH (400 MHz, CDCl3) 2.39 (2H, t, 3J = 7.6 Hz), 2.86 (2H, t, 3J = 7.6 Hz), 3.85 (3H, s, OCH3), 4.31 (2H, d, 3J = 5.6 Hz), 5.09 (2H, s, CH2), 5.56 (1H, bs, NH), 6.70 - 6.73 (2H, m), 6.77 (1H, d, 3J = 8.4 Hz), 7.03 - 7.06 (2H, m), 7.22 - 7.39 (8H, m); δC (100.5 MHz, CDCl3) 31.3 (CH2), 38.6 (CH2), 43.5 (CH2), 56.0 (OCH3), 70.8 (OCH2), 111.8 (CH), 114.3 (CH), 121.0 (CH), 127.4 (2C, CH), 127.6 (2C, CH), 127.5 (Cquat), 127.7 (CH), 128.5 (3C, CH), 128.6 (2C, CH), 133.0 (Cquat), 137.1 (Cquat), 147.9 (Cquat), 148.1 (Cquat), 172.0 (Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 376 (MH+, 11).
N-Benzyl 3-hydroxy-4-methoxyphenylpropionamide (35). -colorless solid, mp. 116˚C - 117˚C (Lit. mp. 117 ˚C [21] ); νmax (KBr/cm−1) 3501 (bs), 3295 (bs), 1636, 1517, 1453, 1276, 1215, 1128, 1024, 862, 805, 696; δH (400 MHz, CDCl3) 2.47 (2H, t, 3J = 7.6 Hz), 2.89 (2H, t, 3J = 7.6 Hz), 3.85 (3H, s, OCH3), 4.39 (2H, d, 3J = 5.6 Hz, NCH2), 5.60 (1H, bs, NH), 6.65 (1H, dd, 3J = 8.4 Hz, 4J = 2.2 Hz), 6.73 (1H, d, 3J = 8.4 Hz), 6.76 (1H, d, 4J = 2.0 Hz), 7.13 - 7.15 (2H, m), 7.24 - 7.30 (3H, m); δH (100.5 MHz, CDCl3) 31.1 (CH2), 38.6 (CH2), 43.6 (NCH2), 56.0 (OCH3), 110.7 (CH), 114.4 (CH), 120.0 (CH), 127.4 (CH), 127.8 (2C, CH), 128.6 (2C, CH), 133.9 (Cquat), 137.5 (Cquat), 145.1 (Cquat), 145.6 (Cquat), 172.0 (Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 286 (MH+, 21).
Benzyl 4-aminobenzoate (37) [22] . -colorless needles, mp. 97˚C; νmax (KBr/cm−1) 3456, 3359, 3223, 2937, 1683, 1632, 1572, 1517, 1436, 1380, 1310, 1278, 1170, 1116, 974, 846, 771, 730, 691; δH (400 MHz, CDCl3) 4.06 (2H, bs, NH2), 5.31 (2H, s, OCH2), 6.62 (2H, d, 3J = 8.8 Hz), 7.30 - 7.44 (5H, m), 7.88 (2H, d, 3J = 8.8 Hz); δC (100.5 MHz, CDCl3) 66.1 (OCH2), 113.8 (2C, CH), 119.6 (Cquat), 128.0 (2C, CH), 128.5 (2C, CH), 131.8 (3C, CH), 136.6 (Cquat), 150.9 (Cquat), 166.5 (Cquat, CO); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 228 (MH+, 10).
3-Aminobenzyl benzyl ether (39) [23] . -as a pale yellow oil; νmax (KBr/cm−1) 3500 (bs, NH), 3369 (NH), 3029, 2855, 1619, 1493, 1358, 1299, 1068; δH (400 MHz, CDCl3) 4.48 (2H, OCH2), 4.55 (2H, OCH2), 6.62 - 6.64 (1H, m), 6.73 - 6.77 (2H, m), 7.14 (1H, dd, 3J = 8.0 Hz, 3J = 8.0 Hz), 7.25 - 7.39 (5H, m); δC (100.5 MHz, CDCl3) 70.0 (OCH2), 70.1 (OCH2), 111.5 (CH), 114.5 (CH), 116.2 (CH), 117.6 (CH), 127.8 (2C, CH), 128.4 (2C, CH), 129.3 (CH), 138.3 (Cquat), 139.5 (Cquat), 146.2 (Cquat); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 214 (MH+, 19).
O-Benzyl 2-aminophenol (44) [24] . -slowly crystallizing oil; mp. 36˚C (Lit. mp. 38˚C [24] ) νmax (KBr/cm−1) 3466, 3378, 3061, 3032, 2927, 2869, 1613, 1504, 1454, 1381, 1276, 1216, 1142, 1042, 1016, 910, 856, 735, 697; δH (400 MHz, CDCl3) 3.60 (2H, bs, NH), 5.09 (2H, s, OCH2), 6.71 - 6.88 (4H, m), 7.32 - 7.42 (3H, m), 7.45 (2H, d, 3J = 7.6 Hz); δC (100.5 MHz, CDCl3) 70.4 (OCH2), 112.1 (CH), 115.3 (CH), 118.5 (CH), 125.5 (CH), 127.6 (2C, CH), 128.0 (CH), 128.6 (2C, CH), 136.4 (Cquat), 137.2 (Cquat), 146.5 (Cquat); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 200 (MH+, 11).
2-Aminophenol (45). -colorless solid; mp. 173˚C (Lit. 174˚C [16] ); νmax (KBr/cm−1) 3377 (NH), 3306 (NH), 1608, 1515, 1475, 1406, 1285, 1271, 900, 751, 745; δH (400 MHz, DMSO-d6) 4.70 (2H, bs), 6.40 (1H, d, 3J = 7.6 Hz), 6.54 - 6.59 (2H, m), 6.65 (1H, d, 3J = 6.8 Hz), 8.70 (1H, bs, OH); δC (100.5 MHz, DMSO-d6) 114.5 (2C, CH), 116.5 (CH), 119.5 (CH), 136.4 (Cquat), 144.0 (Cquat); MS (EI, 70 eV) m/z (%) 109 (M+,100), 80 (32).
3. Results and Discussion
The reagent system was also noted to be effective in C-Cl dechlorination reactions [25] . However, when we tried to use NaBH4, CH3CO2H, Pd/C in O-debenzylation reactions, the O-debenzylation, e.g. from 3 to 4, did not proceed, even with an excess of reagent (Scheme 2).
This result gave the authors an indication that NaBH4, CH3CO2H, Pd/C could be used as a reaction system that would allow to hydrogenate double bonds in the presence of benzyl ethers and benzyl esters. The following describes hydrogenations of alkenes carrying O-benzyl ether, O-benzyl ester and N-benzyl amide functions with this reducing agent.
When a finely ground powder of NaBH4 is added to a mixture of alkene, acetic acid and Pd/C in toluene or benzene, fine gas bubbles appear immediately. The sodium borohydride reacts with the acetic acid, giving, apart from sodium acetate (8), hydrogen and borane. Borane (9), or its formed dimer, diborane (10), would then hydrolyse with the water introduced with the acetic acid and solvents to form boric acid (11) and further hydrogen (Scheme 3). The hydrogen thus produced in situ provides the reagent in the Pd/C catalyzed hydrogenation reaction of the alkenes. The life-time of the borane (9)/diborane (10) produced in the reaction has not been ascertained and so great care must be taken, as borane (9)/diborane (10) are highly toxic, and a complete hydrolysis of the borane with completion of the hydrogenation of the alkenes has not been established.
With NaBH4, CH3CO2H, Pd/C in toluene or benzene, alkenes 12-24, carrying O-benzyl ether functions and/or O-benzyl ester groups could be hydrogenated effectively without loss of the O-benzyl function. Multiple double bonds in a substrate are completely hydrogenated under the conditions as can be seen in the transformation of 22 to 23. Ketones are not reduced with NaBH4, CH3CO2H, (cat.) Pd/C, evident in the conversions of 20/22 to 21/23. Upon careful handling, even a carbaldehyde-function can be retained in the reaction as can be seen in the transformation of 2-benzyloxycinnamaldehyde (24) to 2-benzyloxyphenylpropionaldehyde (25) with only relatively small amounts of 3-(2-benzyloxyphenyl)propanol (26) evident as by-product (Figure 1).
It is known that also ammonia, ammonium acetate and pyridine suppress reductive O-debenzylation with hydrogen in the presence of Pd/C, while the hydrogenation of alkenes proceeds under the conditions [9] . Also, amines have been noted to suppress the reductive cleavage of benzyl ethers [26] -[28] . Momentarily, the mechanistic reasoning behind the suppression of the O-debenzylation in our case is not clear. The accepted mechanism for the Pd(0) hydrogenative O-debenzylation is shown in Scheme 4. It must be noted that the reaction is taken place under heterogeneous conditions, while the mechanism does not take this into account. It is believed that the reductive step D to E is significantly important to determine the character of the “Pd(0)” species and may depend on the reactive system.
Also, N-benzyl cinnamides 27, 29, 31 and 33 were subjected to hydrogenation with NaBH4, CH3CO2H in the presence of cat. Pd/C to afford the corresponding N-benzyl phenylpropionamides 28, 30, 32 and 34 (Scheme 5). Due to the poor solubility of 31 and 33 in toluene or benzene, their hydrogenation was carried out in a mixture of toluene and THF (1/1 v/v). No N-debenzylation was observed in the reactions. Overall, the stability of the benzyl protective group (Bzl) was found to be -NHBzl; -CH2OCH2Ph > PhOCH2Ph > -CO2CH2Ph, under the reaction conditions used. This could be seen when N-benzyl 4-methoxy-3-benzyloxycinnamide 33 was subjected to prolonged reaction with NaBH4, CH3CO2H, Pd/C in a solvent mixture of toluene and THF (1:1 v/v), where the O-benzyl function was reductively cleaved as well to give phenol 35 (Scheme 6).
![]()
Scheme 2. O-Debenzylation of 3 does not proceed under the conditions.
![]()
Scheme 3. Reaction sequences of NaBH4 (7) in presence of acetic acid (6) and water.
![]()
Figure 1. Reduction of alkenes carrying O-benzyl esterand/or O-benzyl ether functions.
Finally, the use hydrogen in presence of Pd/C is an often used method to convert nitroarenes to anilines [29] . Other hydrogen sources such as formic acid and decaborane with Pd/C have been used in the transformation [30] [31] . It was found that nitroarenes are reduced to anilines also with the system NaBH4 and CH3CO2H in the presence of cat. Pd/C. Here, benzyl 4-nitrobenzoate (36) could be converted cleanly to benzyl 4-aminobenzoate (37). Furthermore, benzyl 3-nitrobenzyl ether (38) could be transformed to 3-aminobenzyl benzyl ether (39) (Scheme 7). However, in the case of both benzyl 2-nitrobenzoate (40) and benzyl 2-nitrobenzyl ether (43), the benzyl group was removed reductively to give mixtures of anthranilic acid (42) and benzyl 2-aminobenzoate (41) and of 2-aminobenzyl benzyl ether (44) and 2-aminophenol (45), respectively (Scheme 8). Here, close proximity of the nitro group to the benzyl function leads to partial reductive cleavage of the latter. While the reduction
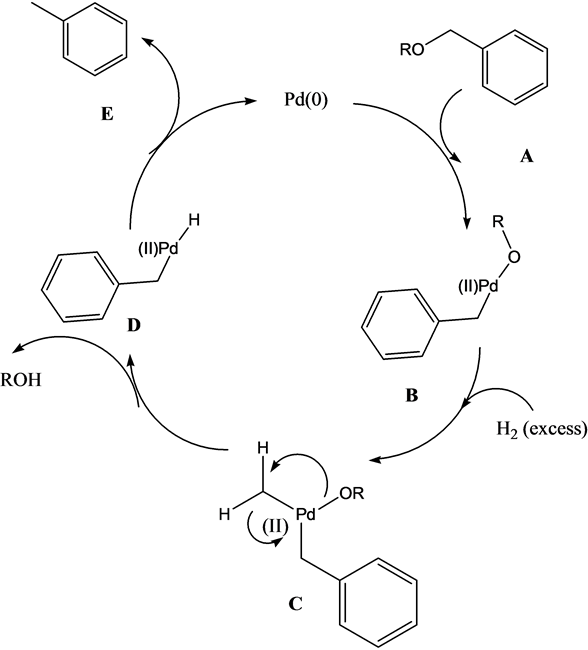
Scheme 4. Accepted mechanism for Pd(0) catalyzed hydrogenative O-debenzylation.

Scheme 5. Hydrogenation of N-benzyl cinnamides to N-benzyl phenylpropionamides.
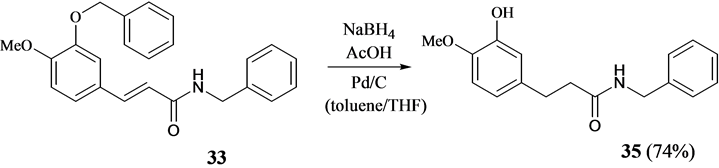
Scheme 6. Concomittant O-debenzylation and alkene hydrogenation of 33 with NaBH4, acetic acid and Pd/C as catalyst.
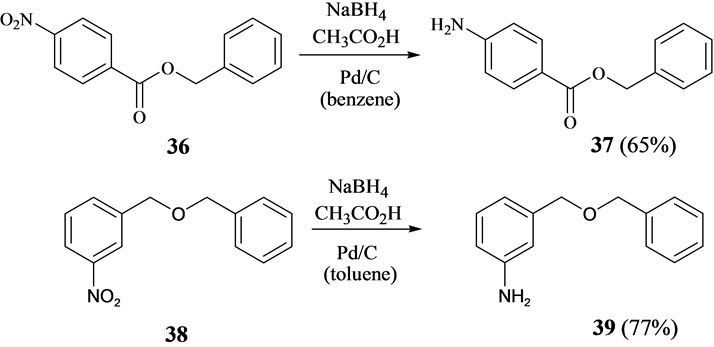
Scheme 7. Reductive transformation of nitroarenes to anilines with NaBH4, acetic acid and Pd/C as catalyst.
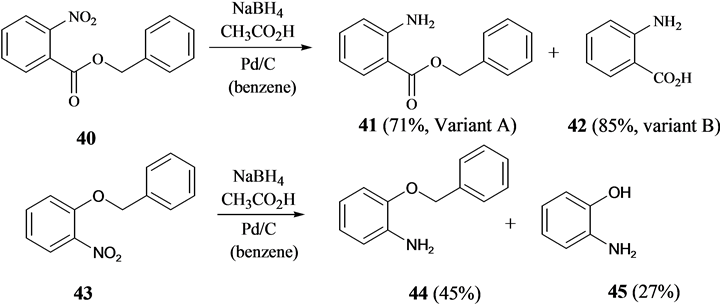
Scheme 8. Reductive transformation of nitroarenes with benzyl functions in close proximity to the nitro group.
of the nitro group can pass through a number of intermediates and can be mechanistically complex, it is believed that a reactive intermediate along the pathway from nitro- to amino-function leads to the reductive cleavage of the benzyl ether in 44 and benzyl ester in 41.
4. Conclusion
With NaBH4, AcOH in the presence of catalytic amounts of Pd/C, a simple reactive system was utilized to hydrogenate alkenes in the presence of O-benzyl ether and benzyl ester protective groups, which are not affected by the reaction. It was found that an aromatic nitro function is reduced to amino group by NaBH4, AcOH, cat. Pd/C. Here a benzyl ether or a benzyl ester function can then be retained, when in the substrate the nitro group and the benzyl function are positioned adequately far apart.
NOTES
![]()
*Corresponding author.