Comparison and Evaluation of Two Analytical Methods for Cation Exchange Capacity and Exchangeable Sodium Percentage of Five Soil Types in Central Sudan ()
Received 22 November 2015; accepted 18 December 2015; published 21 December 2015

1. Introduction
The major soils in Sudan can be divided geographically into three categories: the sandy soils of the northern and west central regions, the clay soils of the central and eastern regions, and the laterite soils of the southern regions.
Most agricultural and environmental planning requires soil analysis, or at least should require analysis for better implementation for any change. Furthermore, better practical analysis methods can rapidly estimate soil properties needed to improve quantitative assessments of land management problems [1] .
The basic problem of this research is that the soil laboratories in the Sudan, do not enter the new modern means of analyzing soil, and most of the methods used are very old. Studies have shown the lack of modern efficiency to meet required needs. There are new methods of analysis, with less expenses and time-consuming, and they have not been used in the Sudan.
CEC is important for maintaining adequate quantities of plant available calcium (Ca2+), magnesium (Mg2+) and potassium (K+) in soils. Under acid conditions (pH < 5.5), aluminum (Al3+) may also be present as an exchangeable cation. While a soil with a higher CEC may not necessarily be more fertile, when combined with other measures of soil fertility, CEC is a good indicator of soil quality and productivity.
The Cation Exchange Capacity is an important property of clay minerals. CEC results are frequently used for characterization and quantification of sorbents in clays and soils. Determination of the CEC and exchangeable cations of soils and clays have been performed since the early work [2] .
Numerous publications about various methods reflect the limitation of analytical validity of result obtained by CEC procedures for the wide variety of natural materials. However, common CEC methods like ammonium acetate [3] or barium chloride [4] , are time consuming, and results for natural materials are often poor. Recent methodological approaches use the higher selectivity of metal-organic complexes, e.g. silver-thiourea method as a one-step procedure compared to common methods to reduce the time factor [5] .
Methods have been proposed for calcareous and gypsiferous soils, [4] [6] - [12] . They are not generally applicable to soils containing both CaCO3 and gypsum or they are too cumbersome and demanding for routine determinations.
In general, analytical problems in CEC methods developed through specific interactions between components of soils and clays with the exchange solutions used. The main sources of errors are soluble (Ca2+) phases and in the faster modern methods, hydrophobic interaction can cause unrealistic CEC values. The objective of this research was to compare and evaluate the results of the [6] and [13] methods for determination of Cation Exchange Capacity (CEC) and Exchangeable Sodium Percentage (ESP) of five soil types in central Sudan.
2. Materials and Methods
2.1. Soil Sampling and Characterization
Twenty-six soil samples were collected from different five soil profiles were dug in central Sudan based on different geographical, climatological and ecological regions, which included; Gedaref area (14°10'64"N 35°38'26"E), Soba area (15°52'66"N 32°60'79"E), Wad Madani area (14°39'19"N 33°49'30"E), College farm (15°64'74"N 32°51'76"E), and recent Nile terrace at Khartoum North area (15°65'16"N 32°51'25"E).
Each soil profile was studied in the field, and described following the format of the FAO [14] , guidelines of soil profile description and sampled according to genetic horizons and classified on the bases of its diagnostic characteristic used at different categories levels of the American system for soil classification [15] .
Each sample was kept in a cloth bag, labeled with; collected data, area, soil profile number, sample depth, then, subjected to physical and chemical analyses at the soil laboratories in Khartoum University. Soil pH was determined on the saturated paste and the electrical conductivity of the saturation extraction was used as a measure of soil salinity. The organic matter (OM) was determined used Walkley-Black method [16] . Texture classes were determined using [17] method. P was analyzed using colorimetric method by spectrophotometer [18] .
Flame photometer was used in sodium measurement, after adjusting the flame photometer by the standard solution (100 ppm Na) at 100 reading and distilled water at Zero reading. Extractable Na was obtained by using a known volume of 1 N ammonium acetate and the exchangeable contents of these elements were obtained by difference between the extractable and soluble quantities. While exchangeable sodium percentage (ESP) was calculated, using results of two CEC methods.
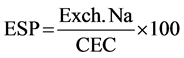
where: Exch. Na = Exchangeable sodium in meq/100g.
CEC = Cation Exchange Capacity meq/100g.
2.2. Determination of Cation Exchange Capacity (CEC)
According to [6] method, 4 g of each soil sample was placed in centrifuge tube, 33 ml of IM sodium acetate was added, and pH adjusted to 8.2. The soil suspension was shaken for 5 minutes and then centrifuged until the supernatant liquid was clear. The supernatant was then discarded completely. The sample was extracted in this manner a total of tour times. Excess salt of sodium acetate washed four times by adding 33 ml 95% ethanol to the tube. The adsorbed sodium replaced by three extractions with 33 ml 1 M ammonium acetate, shacked, centrifuged and supernatant liquid collected in 100 ml volumetric flask. Finally, Sodium concentration was measured used flame photometer.
According to [13] method, 4 g of each soil sample from different profiles was saturated with sodium by four successive equilibrations with 33ml aliquots of a 60% ethanol solution. During each equilibration the soil suspension was shaken for 5 minutes, and then centrifuged for 5 minutes (2000 rpm/min) until the supernatant was clear after which the supernatant liquid was discarded. The Na-saturated samples plus the occluded saturating solution were extracted three times with 33 ml aliquots of 0.5 M pH 7 solution of Mg (NO3)2. The exchangeable Na was calculated as the total Na minus the occluded soluble Na that was 5 times the occluded Cl.
2.3. Statistical Analysis
Statistical differences between samples were determined using statistical analysis [19] , using T-test with multiple samples where differences were calculated from various measurements. The means of these differences were obtained (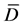 ), the deviation from each measurement was used to get the standard deviation (sd). Then, the T value was calculated from the equation below:
), the deviation from each measurement was used to get the standard deviation (sd). Then, the T value was calculated from the equation below:
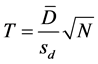
where: T ≡ Calculated T value.
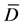 ≡ Means of differences.
≡ Means of differences.
sd ≡ Standard deviation.
N ≡ Number of samples.
3. Results and Discussion
3.1. Morphological Properties
The description of the study sites and selected morphological properties of representative soil profiles are presented in (Table 1 and Table 2), respectively. The parent material of Profiles I and II was alluvium/colluvium and old alluvium of the Bule Nile, respectively. While the parent material of profiles III, V, and VI were alluvium. Soil texture of all profiles belong to five textural classes; loam, sandy clay loam, clay loam silty clay and clay. All profiles showed angular/sub-angular blocky structure in the surface horizon and the lower horizons were massive. The quantity of roots in the soil profiles decreased with depth and the boundary between horizons was generally diffused and smooth.
![]()
Table 1. Selected site properties of the studied profiles.
![]()
Table 2. Selected morphological properties of the representative profiles.
Texturea; C: clay; Scl: Sandy clay loam; Sic: silty clay; Cl: clay loamy; L: loam. Structureb; 1: weak; 2: moderate; 3: strong; f: fine; m: medium; c: coarse; sbk: subangular blocky; abk, angular blocky; ma: massive. Rootsc; 1: very few; 2: few; 3; moderate; 4: common; f: fine; m: medium; c: coarse. Boundaryd; a: abrupt; c: clear; d: diffuse; i: irregular; s: smooth; w: wavy.
3.2. Physical and Chemical Properties
Some of the physical and chemical properties of the representative profiles were presented in (Table 3). Gadarif area soil was non-saline, non-sodic and calcareous, while Soba area soil at Khartoum state was saline-Sodic and calcareous. The soil of Agricultural Research Corporation Farm, Gezira State is non-saline at the depth of 0 - 84
![]()
Table 3. Some physical and chemical properties of the representative profiles.
cm, slightly saline at the bottom depth 84 - 150 cm, sodic and slightly calcareous. The Nile flood plain soil was non-saline, non-calcareous and non-sodic; it was very suitable for agriculture. The soil of Shambat area―Coll- ege of Agricultural Studies Farm is non-saline at the top surface 0 - 83cm, and slightly saline at the bottom 83 - 150 cm, sodic and slightly calcareous soil.
3.3. Determination of Cation Exchange Capacity by (Bower et al., 1952) and (Mario and Rhoades, 1977) Methods
The only method used for determination of Cation Exchange Capacity (CEC) in Sudan is [6] , which is not replaced or changed although errors can result during measurement of CEC. Errors observed during saturation step with adsorb cation, in the washing step of the excess cation with alcohol, and in the decantation step. [13] mentioned that; during saturation step in Bower et al., (1952) the adsorb Na+ could not be complete saturated in the soil, because of the competence of other cation at the exchange sites, e.g. Ca+ which is abundant in calcareous soils. (The study areas of Gedaref, Gezira contained high amount of CaCO3), these areas the most important agricultural areas in Sudan, so accurate results for CEC should be obtained.
During washing step, adsorb Na+ may be lost by hydrolyses and replaced by other cations (e.g. Ca+ and gypsum) [10] [13] [20] . Probably, the significant differences at Gedaref and Wad Medani areas when used two methods are due to the above-mentioned reasons.
[13] also mentioned that the loss of organic matter from the soil at the washing step using alcohol, or at the decantation step where some clay minerals could lost, which have a significant role in CEC, especially two layers clay minerals. In this study, the soil samples are poor in organic matter, so what have been mentioned of these lost not affect in the results obtained. Two layers clay minerals (e.g. montmorillonitic, Na+ ions may trapped between the two layers could not be replaced or washed by alcohol, leads to wrong CEC reading). Soil rich in zeolits and feldspars, and vermiculites assists Na+ to be trapped at the center extraction [13] . This can affect the CEC readings and spontaneously affect exchangeable sodium percentage (ESP).
[13] method alleviate many errors that could occur in applying Bower method, plies its simplicity and more or less accuracy in calcareous and gypsiferous soils. In addition, [13] omitted the washing step to remove excess sodium. Figure 1 showed the differences in CEC and ESP of the two methods.
Significant differences appeared in Gedaref area, Wad Medani and Nile flood plain when used these two methods for determination CEC (Table 4). Significant differences also appeared in ESP at Nile flood plain and Shambat area (Table 5), that may be due to; effect of several washing solution lead to significant dissolution of O.M, which lowered the CEC systematically, agreed by [21] . The permanently added Ca2+ ions compete successfully with 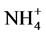 during the washing step lead to underestimation of CEC [13] . High layers charge density allows slow cation exchange when
during the washing step lead to underestimation of CEC [13] . High layers charge density allows slow cation exchange when 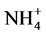 was used.
was used.
![]()
Figure 1. The difference between results by using (Bower et al., 1952) and (Mario and Rhoades, 1977) methods for determination of CEC and ESP.
![]()
Table 4. Statistical comparison between CEC results using (Bower et al., 1952) and (Mario and Rhoades, 1977) methods.
N.SNon significant. *Significant at (P > 0.05).
![]()
Table 5. Statistical comparison between ESP results using (Bower et al., 1952) and (Mario and Rhoades, 1977) methods.
N.SNon significant. *Significant at (P > 0.05).
Yaalon et al., (1962) reported that, the use of 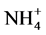 salts should be avoided because of the possibility of NH4 fixation and release of fixed potassium resulting in underestimation of CEC.
salts should be avoided because of the possibility of NH4 fixation and release of fixed potassium resulting in underestimation of CEC.
It can be stated that, the newly developed method of Mario and Rhoades used in this study is more practical, simple and reliable for determination of CEC as the currently used Bower method, but will be safer than Bower method in some problematic soils. The adoption of Mario and Rhoades method is advisable because it is less time consuming as it omitted the washing step. It cannot be a good substitute in laboratories which no possibility to determination sodium by flame photometer.
4. Conclusion
After compared and evaluated of Bower et al., (1952) and Mario and Rhoades (1977) methods for determination of Cation Exchange Capacity (CEC) and Exchangeable Sodium Percentage (ESP), we conclude that when Mario and Rhoades (1977) method is used to determine CEC and ESP time will be saved, that less amount of chemicals will be used in laboratories and that accurate results will be achieved.
Acknowledgements
Special thanks to the staff of the laboratories of the Land and Water Research Centre at the Agricultural Research Corporation, Wad Medani, and the Staff of Soil Science and Water Department at College of Agricultural Studies, Sudan University of Science and Technology, for availing their laboratories facilities and for their technical advice.
NOTES
![]()
*Corresponding author.