Spectrofluorometric Assays of Human Collagenase Activity Using Native Collagen and Acetyl-Peptide Substrates ()
1. Introduction
Matrix metalloproteinases (MMPs) are a family of zinc-dependent enzymes that degrade major proteins in the extracellular matrix [1] . Human collagenase is a MMP with the ability to cleave triple-helical native collagens at a single site, resulting in two large fragments that are approximately 1/4 and 3/4 of the initial length [2] . Human collagenase-1 (MMP-1), collagenase-2 (MMP-8), and collagenase-3 (MMP-13) have been well-characterized physiologically [3] .
MMP-13 degrades several fibrillary collagens such as Ι, ΙΙ, ΙΙΙ, ΙV, IX, and X [4] . Elevated gene expression of this enzyme has been observed in patient tissues having malignancies such as adenocarcinoma, squamous cell carcinoma, and basal cell carcinoma [4] . This enzyme is also involved in the pathogenesis of rheumatoid arthritis because it is significantly elevated in the synovial fluid and serum in such patients [5] [6] .
Several assays for human collagenase activity use radioisotope-labeled [7] -[10] or fluorescence (FL)-labeled [11] collagens or peptides as substrates. Additionally, several specific anti-collagenase antibodies have been developed for MMP detection and for screening MMP inhibitors [12] -[14] . Even though these human collagenase assays are facile, either enzyme- or FL-labeled antibodies or substrates are required, thus precluding label-free substrates.
We have developed several inexpensive fluorogenic reactions using non-FL organic reagents for the highly sensitive detection of specific oligopeptides. The FL reagents can selectively label a few peptides because they react only with the N-terminal amino acids. For example, hydroxylamine in the presence of cobalt(II) and borate selectively labels N-terminal, Tyr-containing peptides [15] . Other selective FL reagents include glyoxal for N- terminal Trp-containing peptides [16] , 1,2-dihydroxybenzene(DHB) and borate for N-terminal Phe-, Ile-, Leu-, Val-, or Ala-containing peptides [17] , DHB and 2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid buffer at pH 7.5 for N-terminal Ser-containing peptides [18] , 3,4-dihydroxyphenylacetic acid (DHPAA) for N-terminal Gly-containing peptides [19] , and 3,4-dihydroxybenzoic acid for N-terminal Pro-containing peptides [20] . These reagents could be used to analyze several peptides in complex mixtures such as tissues and enzymatic digests [18] [21] -[24] .
Previously [24] , we reported sensitive quantification of human collagens by using the fluorogenic reagent, DHPAA for labeling N-terminal Gly-containing oligopeptides after enzymatic digestion by bacterial collagenase. Here, we used DHPAA to sensitively detect the activity of human collagenase because the enzyme cleaves collagens into large fragments. The fragments are separated from unreacted collagen substrate by ethanol extraction, and their production is quantitatively determined by the DHPAA-based FL reaction for N-terminal Gly-con- taining peptides. The latter peptides can be generated by continued enzymatic digestion of the fragments with excess bacterial collagenase. We have also examined DHB fluorogenic reaction [17] with N-terminal Ile-con- taining peptides and an acetyl peptide (Ac-GPQGIAGQ), which was reported to be a substrate [25] for human collagenase. Overall, we describe optimized assay protocols for human collagenase activity using the fluorogenic reagents DHPAA and DHB, and discuss the activity of endogenous collagenase in human cells and tissues.
2. Materials and Methods
2.1. Materials
Human placental collagens Ι and ΙV and bovine nasal septum collagen ΙΙ were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gly-Pro (GP) was obtained from Wako Pure Chemicals (Osaka, Japan) and dissolved in water. Ac-GPQGIAGQ and Ac-GPQAIAGQ were obtained from Sigma-Aldrich. Recombinant human activated collagenase-3 (MMP-13; 100μg/mL solution in 0.05 M Tris-HCl buffer at pH 7.6 containing 0.2 M NaCl, 5.0 mM CaCl2, 20 μM ZnSO4, 0.1% bovine serum albumin and 0.0025% NaN3) was purchased from Chondrex Inc. (Redmond, WA, USA). Clostridium hystolyticum collagenase was purchased from Nacalai Tesque (Kyoto, Japan). DHPAA and DHB were purchased from TCI (Tokyo, Japan). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Wako Pure Chemicals. Fetal bovine serum (FBS) was purchased from Gibco- Invitrogen (Grand Island, NY, USA). PSA (100 units/mL penicillin, 0.1 mg/mL streptomycin and 0.25 μg/mL amphotericin B) was purchased from Nacalai Tesque. Water was purified with a Milli-Q system WR600 A from Millipore (Molsheim, France). All other chemicals were of analytical or guaranteed-reagent grade, and used without further purification.
2.2. Preparation of Substrate and Enzyme Solutions
Human collagen ΙV and acetyl-peptide substrates were dissolved in water. Human collagen Ι and bovine collagen ΙΙ were dissolved in 0.5 M acetic acid at 37˚C and then neutralized to pH 6.5 - 7.5 using 0.5 M NaOH. These substrate solutions were stored at 4˚C, and used within 1 month. MMP-13 enzyme was stored at −80˚C until use. The bacterial collagenase (100 μg/mL) was dissolved in a solution of 125 mM borate buffer (pH 7.5) and 10 mM CaCl2, and stored at −20˚C.
HeLa cells or fibroblast cells from normal human skin were cultured in a 10-cm dish, and allowed to grow to 80% - 90% confluence in PSA and DMEM containing 10% FBS by incubating at 37˚C in a humidified atmosphere of 5% CO2 and 95% air. Cells were removed from the dish with trypsin after the second passage (5 - 6 days). After washing once with phosphate-buffered saline solution (PBS), the cells were stored at −80˚C until analysis. The cells (106 - 107 cells/mL) were lysed by repeated (3×) 10-min sonications at 4˚C in a 125 mM borate buffer (pH 7.5) containing 10 mM CaCl2. The lysate was centrifuged at 12,000 g for 10 min and the supernatant was separated from the pellet, which was then suspended in 125 mM borate buffer (pH 7.5) containing 10 mM CaCl2. The supernatant (cytosol) and the pellet (cell debris) were stored at −80˚C and used as enzyme sources. Normal human (30 years old) cheek tissue from buccal mucosa was collected with a cotton swab for 2 - 3 min and suspended in PBS. After washing by centrifugation at 1000 g for 5 min, the precipitated tissue was suspended in 125 mM borate buffer (pH 7.5) containing 10 mM CaCl2 at 4˚C, and then lysed by repeating (3×) 10-min sonications. The lysate was centrifuged at 12,000 g for 10 min and the supernatant was separated from the pellet. The pellet was suspended in 125 mM borate buffer (pH 7.5) containing 10 mM CaCl2, and the supernatant (cytosol) and pellet (cell debris) were stored at −80˚C and used as enzyme sources. Total protein content in the cells and tissue fractions were measured with a Quick-Start Bradford Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA).
2.3. Activation of Endogenous Collagenase in Biological Specimens
Collagenase in human cells or tissue is generally present in an inactivated form and thus needs to be activated as follows [26] . A 100-μL aliquot of each enzyme source was mixed with 80 μL of 125 mM borate buffer (pH 7.5) containing 10 mM CaCl2. Then, a portion (10 μL) of 43 μM trypsin was added and the solution was incubated at 37˚C for 10 min. After collagenase activation, a portion (10 μL) of 3.0 mg/mL soybean trypsin inhibitor was added. For MMP-13, this activation was not necessary.
2.4. Assay Protocol for Collagenase Activity Using Collagen Substrate and DHBA FL-Reagent
A 25-μL aliquot of 740 nM MMP-13, or another enzyme source, was mixed with 75 μL of 125 mM borate buffer (pH 7.5) containing 10 mM CaCl2; the enzymatic reaction was then initiated by adding 100 μL of 3.3 μM collagen substrate followed by incubation at 37˚C for 3 h. For negative controls, the enzymatic reaction was performed either without the collagen substrate or the enzyme source. After the reactions, a portion (200 μL) of 99.5% (w/w) ethanol was successively added and thoroughly mixed. The solution was kept in an ice-water bath (0˚C - 4˚C) for 20 min, and then centrifuged at 9300 g at 4˚C for 10 min. The supernatant (20 μL) containing large peptide fragments from the collagen substrate was mixed with 130 μL of 125 mM borate buffer (pH 7.5) containing 10 mM CaCl2. By adding 50 μL of 1.0 μM bacterial collagenase, and then the mixture was incubated at 37˚C for 1 h to produce abundant N-terminal Gly-containing oligopeptides. After that incubation, a 40-μL aliquot of the reaction mixture was sequentially mixed with 20 μL of 250 mM borate buffer (pH 7.5), 20 μL of 0.75 mM DHPAA, and 20 μL of 1.26 mM NaIO4, and then immediately incubated at 37˚C for 10 min for FL labeling. After the incubation, the final mixture was cooled in an ice water bath to stabilize the FL product. The FL intensity at 465 nm was measured with a spectrofluorometer (FP-7200, Jasco, Tokyo, Japan) during 375-nm excitation in a 3-mm quartz micro-cell. All measurements for each sample were performed in duplicate.
2.5. Assay Protocol for Collagenase Activity Using FITC-Collagen Substrate
A commercially available assay kit containing FITC-labeled bovine collagen I (Chondrex Inc. Redmond, WA, USA) was used for a comparison with the new assay methods. According to the manufacturer’s protocol, a portion (50 μL) of 740 nM MMP-13 or other enzyme sources was mixed with 150 μL of solution B (unknown composition). Enzymatic reaction was initiated by mixing with 200 μL of 1.0 mg/mL FITC-labeled bovine collagen I substrate, followed by incubation at 37˚C for 3 h. For negative controls, the enzymatic reaction was performed either without the collagen substrate or the enzyme source. To stop the enzymatic reaction, 10 μL of 10 mM o-phenanthroline and 10 μL of 38.5 μM elastase were added to every sample, followed by incubation at 37˚C for 10 min. Finally, a 400-μL aliquot of extraction buffer (unknown composition) was mixed well with the reaction solution, which was then centrifuged at 9300 g for 5 min. The supernatant (100 μL) was used for the measurement of FL intensity at 520 nm with the spectrofluorometer during 490-nm excitation in the 3-mm quartz micro-cell. All measurements were performed for each sample in duplicate.
2.6. Assay Protocol for Collagenase Activity Using Acetyl-peptide Substrate and DHB FL-Reagent
A 10-μL aliquot of 74 nM MMP-13 or of other enzyme sources was mixed with 27 μL of 125 mM borate buffer (pH 7.5) containing 10 mM CaCl2, and the enzymatic reaction was initiated by adding 3 μL of 20 mM Ac-GPQGIAGQ substrate, followed by incubation at 37˚C for 3 h. For negative controls, the enzymatic reaction was performed either without the collagen substrate or the enzyme source. The reaction mixture (40 μL) was sequentially mixed with 20 μL of 250 mM borate buffer (pH 7.5), 20 μL of 2.5 mM DHB, and 20 μL of 2.5 mM NaIO4, and immediately heated at 100˚C for 5 min for FL labeling. After the reaction, the final mixture was cooled in an ice water bath to stabilize the FL product. The FL intensity at 500 nm was measured with the spectrofluorometer during 385-nm excitation in the 3-mm quartz micro cell. All measurements for each sample were performed in duplicate.
3. Results and Discussion
3.1. Principle of Collagen Substrate Assay
Collagens have a unique amino acid sequence with approximately 30% Gly residues in each molecule, with a repeated -Gly-X-Y sequence in which Pro and Hyp frequently appear at the X or Y positions [27] . The bacterial collagenase cleaves collagens at the N-terminal of the Gly residue [28] [29] . Therefore, abundant N-terminal Gly-containing oligopeptides are produced from one molecule of collagen [24] .
As shown in Scheme 1, human collagenase generally cleaves collagens at only Gly-Leu or Gly-Ile binding sites, thus producing large peptide fragments [2] . Here, we extracted these fragments from unreacted collagen IV substrate by centrifugation with ethanol, and then enzymatically degraded them to abundant N-terminal Gly- containing oligopeptides by the bacterial collagenase reaction. Thus we amplified the assay sensitivity for human collagenase activity. Finally, the DHPAA FL-reagent [19] was used to selectively label the N-terminal
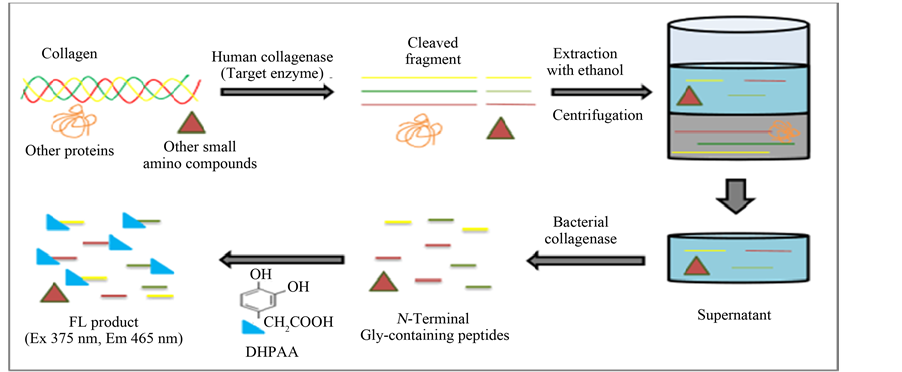
Scheme 1. Protocol for a spectrofluorometric assay of human collagenase activity using collagen substrate. A human collagen IV substrate was hydrolyzed by a small amount of human collagenase (target enzyme) into large fragments. These fragments were then completely degraded by an excess of bacterial collagenase, producing N-terminal Gly-containing peptides. The peptides were selectively labeled by a DHPAA-based FL reaction. Relative fluorescence intensity (RFI) was measured at excitation (Ex) and emission (Em) wavelengths of 375 nm and 465 nm, respectively.
Gly-containing oligopeptides. This DHPAA-based FL reaction can be performed in a neutral borate solution (pH 7.5) at 37˚C for 10 min in the presence of periodate.
3.2. Assay of MMP-13 Activity
MMP-13 cleaves triple-helical collagen at Gly-Ileu or Gly-Leu sites, producing large peptide fragments [2] . Here, the affinities of human collagen Ι, human collagen ΙV, and bovine collagen ΙΙ were tested with regard to the MMP-13 enzyme. As shown in Figure 1(a), human collagen ΙV was more likely to be digested by MMP-13. Thus, the MMP-13 activity assay is based on digestion of the collagen IV substrate.
Because ethanol is a de-proteinization agent, peptide fragments produced by the enzymatic reaction were extracted with ethanol and separated by centrifugation not only from unreacted collagen but also from other endogenous proteins by centrifugation. As shown in Figure 1(b), the background signal from excess substrate was significantly reduced by extraction with 33% - 50% ethanol. Therefore, 50% ethanol was used in enzyme reaction mixtures.
For sensitive detection of human collagenase activity, we tried to produce, via enzymatic reaction with bacterial collagenase, abundant N-terminal Gly-containing oligopeptides from the large fragments extracted in the supernatant following MMP-13 collagen digestion. However, 50% ethanol in the supernatant would inhibit the bacterial collagenase activity. Fortunately, ethanol concentrations < 10% did not inhibit the bacterial collagenase. Thus, we determined the optimum concentration of bacterial collagenase for the second enzymatic reaction mixture in the presence of 2.5% ethanol, as shown in Figure 2(a). The results indicated that 250 nM bacterial
![]() (a)
(a)![]() (b)
(b)
Figure 1. (a) Degradation of collagen substrates (1.65 μM each) by incubation with MMP-13 enzyme (92.5 nM) at 37˚C for 3 h; (b) Effect of ethanol concentration as a de- proteinization reagent for the extraction of fragments produced from the collagen IV substrate. After the enzymatic reaction, ethanol at a concentration ranging from 0% to 66% (v/v) was added to the mixture, followed by centrifugation. Error bars represent standard deviation in duplicate measurements.
![]() (a)
(a)![]() (b)
(b)
Figure 2. (a) Effect of bacterial collagenase concentration for production of N-terminal Gly-containing oligopeptides at 37˚C for 1 h, after the production of large peptide fragments from 1.65 μM human collagen ΙV by 92.5 nM MMP-13 at 37˚C for 3 h; (b) Calibration curve for GP in the enzymatic reaction mixture, which was determined by FL labeling with 0.15 mM DHPAA at 37˚C for 10 min in the presence of 0.25 mM NaIO4 and 50 mM sodium borate (pH7.5). Error bars represent standard deviation in duplicate measurements.
collagenase provided the maximum production of N-terminal Gly-containing peptides from the large fragments. Conveniently, this enzymatic reaction can be performed at 37˚C for 1 h in the same buffered solution as that used for the human collagenase reaction.
Various concentrations (0 - 790 nM) of an N-terminal Gly-containing dipeptide (GP) in the second enzymatic reaction mixture were FL-labeled by the DHPAA reaction in the presence of collagen IV, MMP-13, ethanol, bacterial collagenase, and buffer. As shown in Figure 2(b), the DHPAA yielded FL signals that were proportional to the GP concentrations in the enzymatic reaction mixture
Under conditions described above, 1.65 μM human collagen ΙV was hydrolyzed with 92.5 nM MMP-13 at 37˚C over a range of incubation times (0 - 3 h) and MMP-13 concentrations (0 - 185 nM, at 37˚C for 3 h). As shown in Figure 3(a), the enzymatic products from collagen IV digestion increased with incubation time. Therefore the optimal incubation time of 3 h was determined. Additionally, the FL signals were proportional to enzyme concentrations ranging from 33 - 185 nM, as shown in Figure 3(b). This calibration curve showed a proportional relation (R2 = 0.978) between concentrations of MMP-13 and the FL intensities. The lower limit of detection at 3 of ratio of signal per noise (S/N = 3) was 85 nM MMP-13.
3.3. Comparison with Other Assays
The collagen IV assay discussed above was used to determine human collagenase activities in several enzyme sources such as HeLa cells (107 cells/mL), skin fibroblast cells (106 cell/mL), and cheek tissue from buccal mucosa collected with a cotton swab. As shown in Figure 4(a), we found no significant collagenase activity in the
![]() (a)
(a)![]() (b)
(b)
Figure 3. (a) Kinetics of the enzymatic degradation of 1.65 μM collagen IV by 92.5 nM MMP-13; (b) Calibration curve of 0 - 185 nM MMP-13 using 1.65 μM collagen IV. Error bars represent standard deviation in duplicate measurements.
human samples.
We then used a commercial human collagenase assay kit containing FITC-labeled bovine collagen I to test for collagenase activities in the same samples (Figure 4(b)). In this case, FITC-labeled large fragments were produced by the enzymatic reaction with human collagenase. After the reaction, the FL fragments were degraded by elastase, extracted from the unreacted FITC-labeled substrate, and measured by the spectrofluorometer. However, no collagenase activity was observed by this method. This FITC-labeled collagen method indicated a lower detection limit of 342 nM MMP-13 at S/N = 3. Furthermore, the FITC-labeled collagen substrate was unstable and decomposed during the 3-h enzymatic reaction.
Thus, our assay method using the substrate of human collagen IV for the assay of MMP-13 was approximately 4 times more sensitive than the FITC-labeled collagen method.
3.4. Collagenase Activity Using Acetyl-Oligopeptide Substrate
Human collagenase cleaves the collagen sequence-based oligopeptide GPQGIAGQ at the Gly-Ile site, producing N-terminal Ile-containing IAGQ peptide [12] . Here, we used Ac-GPQGIAGQ as substrate for an assay of human collagenase activity. IAGQ was selectively FL-labeled with a DHB reagent, as shown in Scheme 2. Previously [18] , we evaluated the FL-labeling reactivity of DHB with N-terminal Phe-, Leu-, Ile-, Val- or Ala-containing oligopeptides. This reaction is performed at 100˚C for 5 min in the presence of sodium borate (pH 7.5) and periodate.
Using this assay, the FL signals were proportional to the MMP-13 enzyme concentrations (9.25 - 148 nM), with R2 = 0.999, as shown in Figure 5(a). The lower detection limit of MMP-13 at S/N = 3 was 25 nM. This assay using acetyl-oligopeptide substrate was more sensitive and thus used to measure the enzyme activities in
![]() (a)
(a)![]() (b)
(b)
Figure 4. (a) Collagenase activities using 1.65 μM collagen IV in cytosol (S) and cell debris (P) fractions of HeLa cells, fibroblast cells, and cheek tissue, as measured by the assay method discussed in the text; (b) Collagen activities in the same enzyme sources used for (a), measured with a FITC-labeled bovine collagen I substrate. The concen- tration of total proteins in each specimen is noted in parentheses. Error bars represent standard deviation in duplicate measurements.
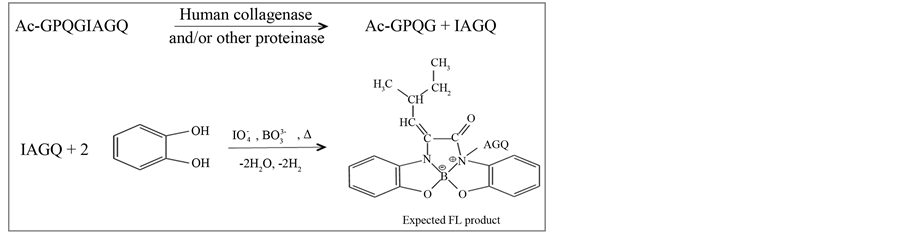
Scheme 2. Protocol for human collagenase assay using an acetyl-pep- tide substrate. The Ac-GPQGIAGQ substrate was hydrolyzed by human collagenase, producing IAGQ peptide that was selectively labeled with a DHB- based FL reaction.
the same biological sources discussed in Figure 4. As shown in Figure 5(b), intense activities were observed in cytosol fractions of cultured cells, in contrast with the negative results using collagen substrates (Figure 4).
4. Conclusions
A selective, sensitive, and convenient assay for human collagenase activity using native collagen or synthetic
![]() (a)
(a)![]() (b)
(b)
Figure 5. (a) Calibration of 0 - 148 nM MMP-13 activities using 1.5 mM Ac-GPQGIAGQ substrate; (b) Collagenase activities in the cytosol (S) and cell debris (P) fractions of HeLa cells, fibroblast cells, and cheek tissue, as measured by the Ac-GPQGIAGQ substrate assay. The total protein concentration in each sample is given in parenthesis. Error bars represent standard deviation in duplicate measurements.
acetyl peptides was presented. The assay was based on selective fluorogenic labeling by either DHPAA or DHB reactions with peptide fragments cleaved from the substrate in the presence of borate and periodate. The collagenase assay conditions for MMP-13 enzyme activity were optimized and any label-free substrates can be used. Thus, various endogenous protease activities could be characterized by this assay.
When the assay was used to measure endogenous collagenase activity in crude biological specimens, such as human cultured cells and cheek tissue, their enzyme sources did not exhibit significant activities for either the collagen substrate (Figure 4(a)) or for commercial FITC-labeled bovine collagen I (Figure 4(b)). These results were probably explained by the low concentrations of collagenase in the samples; endogenous enzyme expression was generally very low and requires stimulation such as aging and/or UV light irradiation [30] [31] . In contrast, when the sequence-based acetyl-peptide substrate was used, intense collagenase activity was observed in the cytosol fractions of cultured cells (Figure 5(b)), which suggested the presence of other endogenous proteases or peptidases. Consequently, this assay would be useful for studies of collagen catabolism.
Acknowledgements
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and was partly supported by the Global Center of Excellence Program of Nagasaki University.
NOTES
*Corresponding author.