Effect of Composition and Morphology on Sensor Properties of Aerosol Deposited Nanostructured ZnO+In2 O3 Films ()
1. Introduction
Metal oxide semiconductor films are widely applied in gas sensors. The properties of such sensors including sensitivity, selectivity and response time depend critically on the film microstructure [1] . The structure in turn is largely determined by the synthesis. Such films are usually synthesized by a variety of techniques including Spray Pyrolysis Technique (SPT) [2] , sol-gel technique [3] , and chemical vapor deposition [4] .
A number of studies have shown that one promising approach to improve conductometric metal oxide sensors is to utilize semiconducting nanostructured composite materials consisting of metal oxides with different electronic structure and chemical properties [5] -[7] . It has been established that using a mixture of metal oxides, the resulting composite sensor material can achieve selectivity and sensitivity for gas detection in air ambience that far exceed those achievable with the individual constituent metal oxides of the composite [8] -[12] .
Studies of sensory phenomena in metal oxide composites have shown that there are certain optimum compositions for which these effects reach maximum values [9] [10] . One of the important factors that determine the dependence of sensory phenomena in metal oxide composites on composition is the effect of composition on the morphology of the sensor film. Therefore, it is important to clarify the morphological features of the composite sensors that exhibit high sensitivity. These features depend on the nature of the components of the mixed metal oxide composite and the processing conditions.
Of particular interest are composite sensor films processed by SPT due to the inherent advantages of the technique, including ease of control, low cost, and simplicity [13] [14] . The present study focuses on SPT-synthe- sized composite films of ZnO + In2O3 and comparison with similar films of ZnO and In2O3. It also relatesthe morphology of the ZnO + In2O3composite films to the sensor response.
2. Experimental Setup and Procedure
Water solutions of zinc chloride and indium nitrate were used to deposit ZnO and In2O3 films by SPT. The ZnO + In2O3composites were synthesized by deposition of mixed solutions of different ratios between the concentrations (C) of the zinc chloride and indium nitrate precursors. The underlying chemical reactions of the SPT deposition can be expressed thus [15] :

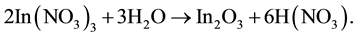
The SPT setup utilized is illustrated in Figure 1 [16] . A precursor solution which contains the constituent reactant compounds is atomized in a nozzle to tiny droplets which are then sprayed onto a preheated alumina substrate. The solutions are injected through a 1 mm-diameter round spray needle and then atomized at 1 bar air pressure [17] . It is important that the temperature of the substrate be maintained within a range that can initiate chemical reaction in the droplet. The droplet also must still contain enough reactants in solution after reaching the surface [18] . A film of stable compounds subsequently forms and adheres to the substrate due to chemical reaction and thermal decomposition of the solution.
The distance between the spray nozzle and the hot surface is kept constant at 10 cm during the experiments. This distance was chosen based on the results of a previous study [16] [19] . Finally, the thin film is annealed for 30 minutes after deposition to the desired structures at 450˚C. The annealing process promotes adhesion of the film to the substrate. These conditions are kept constant for all other sets of experiments performed.
3. Morphology of Aerosol Deposited Sensor Films
The XRD patterns indicate that the SPT-synthesized ZnO films exhibit a polycrystalline hexagonal wurtzite structure while In2O3 films have a polycrystalline cubic structure [10] . The XRD data also indicate that SPT- synthesized ZnO + In2O3composites consist of mixtures of these crystal structures.
The deposited thin films were also characterized by Scanning electron microscopy (SEM). Specifically, the surface morphology was characterized using a Zeiss ULTRA-55 FEG SEM system which used Schottky field emission source and STEM detector. The ZnO films were synthesized at different temperatures. Figure 2 shows the SEM results at 350˚C indicating that at this temperature several small spherical particles are formed and agglomerate at the surface in the shape of powders with an average size of about 50 nm.
Keeping the concentration constant and increasing the deposition temperature, the results (not shown) indicate that dense ZnO oxide film is synthesized by the aerosol deposition at 400˚C. A further increase in temperature to 450˚C increases the film uniformity, but reduces its specific surface area which can adversely affect the sensor response of the film.
Figure 3 shows the SEM micrographs of ZnO thin films synthesized at C = 0.2 mol/lit and C = 0.3 mol/lit. The results show that the grain size of the ZnO film increases with increase in the amount of precursor dissolved in solution.
The SEM micrograph for In2O3 thin films deposited on Al2O3 by SPT at 400˚C and precursor concentration C = 0.3 mol/lit is presented in Figure 4. It is evident that the particles of both the ZnO film shown in a previous Figure 3(b), as well as similar particlesof the SPT-synthesized In2O3 film (Figure 4) are not separate crystallites, but are splices or aggregates of crystallites. An analogous structure has been observed for the particles of SnO2- based nanoheterogeneous film in a previous study [20] .
Thus, there are interparticle contacts of two types in the synthesized films: contacts between crystallites inside crystalline aggregates and contacts between aggregates. The contacts between aggregates of crystallites largely determine the conductivity of the nanocrystalline film [20] .
![]()
Figure 1. Schematic sketch of chemical spray pyrolysis process.
![]()
Figure 2. SEM micrographs of ZnO thin films synthesized by spray pyrolysis on Al2O3 substrate at 350˚C and precursor solution concentration C = 0.1 mol/lit.
![]()
![]() (a) (b)
(a) (b)
Figure 3. SEM micrographs of ZnO thin films on Al2O3 substrate at T = 400˚C and different concentrations of precursor: (a) C = 0.2 mol/lit and (b) C = 0.3 mol/lit.
![]()
Figure 4. SEM micrographs of In2O3 thin films on Al2O3 substrate at T = 400˚C and C = 0.3 mol/lit.
Our study of SPT-processed ZnO + In2O3composite oxide films has demonstrated that the morphology of the films depends strongly on the composition. Figure 5 presents the photomicrographs of the composite films with various ZnO to In2O3 ratios. The composite film 0.25ZnO + 0.75In2O3 (Figure 5(a)) consists of elongated particles of arbitrary shape, which are aggregates that combine small crystallites of In2O3 and ZnO. The composite 0.8ZnO + 0.2In2O3 (Figure 5(b)) contains for the most part faceted particles that are large individual ZnO crystals (or splices of such crystals).In this composite, such particles of ZnO are mixed with the In2O3 particles, which other studies [10] have shown to also increase the sensory response by about 15-20% compared to the response of pure ZnO, i.e. considerably less than in the composite of Figure 5(a).
4. Sensor Properties of Deposited Mixed Films
As indicated above, the particles in the films considered are crystal aggregates, which in the case of the composite sensor consist of a mixture of nanocrystals of In2O3 and ZnO closely interacting with each other. In order to describe the sensor phenomenon, it is necessary to first consider the concentration of conduction electrons and the sensory effect in an isolated nanocrystal line aggregate of the film.
The conductivity of the isolated composite aggregate of ZnO + In2O3 is determined by the In2O3nanocrystals, since the concentration of conduction electrons in the ZnO crystals (1016 - 1017 cm−3) [21] is three to four orders of magnitude lower than in the In2O3 crystals. The concentration of conduction electrons in the In2O3 crystals drops sharply under the influence of atmospheric oxygen, which is chemisorbed on the surface of the crystals, capturing electrons from the conduction band of In2O3 to form O− anion radicals [22] . Sensory response is mani-
![]()
![]() (a) (b)
(a) (b)
Figure 5. SEM micrographs of ZnO + In2O3 thin films on Al2O3 substrate at ratios of (a) 0.25ZnO + 0.75In2O3 and (b) 0.8ZnO + 0.2In2O3 at T = 400˚C and total concentration of two precursors 0.1 mol/lit.
fested as an increase in conductivity due to the sensor reaction of the chemisorbed oxygen anions with hydrogen and the corresponding return of the electrons to the conduction band. The sensory reaction proceeds with participation of the H atoms [23] , formed by the catalytic dissociation of molecular hydrogen.
A characteristic of the sensor response of ZnO + In2O3 aggregates to hydrogen in air is that in these composite aggregates, the In2O3 nanocrystals having high concentration of the conduction electrons (about 1019 - 1020 cm−3) [24] , are in contact with the ZnOn anocrystals, which exhibit high catalytic activity in the reaction of hydrogen dissociation [25] . The contacts between In2O3 and ZnO crystals in a composite aggregate provide particularly favorable conditions for the sensor response of the aggregate to hydrogen in air: dissociation of hydrogen proceeds at the surface of the ZnO crystals with high catalytic cactivity in this reaction while the Hatoms formed on the surface of these crystal sreact with the O− onthesurfaceofIn2O3crystals, thereby increasing the concentration of conducting electrons in the aggregate.
The effect of ZnO on sensory response reaches a maximum value at ZnO composition of about 20%, when almost all of the In2O3crystallites are in contact with the ZnO crystallites [10] . The 0.25ZnO + 0.75In2O3 composite film (Figure 5(a)) has demonstrated sensor response to hydrogen in air close to the maximum, which is 1.7 times the sensor response of pure ZnO films and 4 times the sensor response of pure In2O3 films [10] . A further increase in the composition of ZnO decreases the sensor response due to reduction of the concentration of conduction electrons in the ZnO + In2O3composite.
Under the influence of ambient hydrogen, the equilibrium concentration of conduction electrons inside the isolated ZnO + In2O3 composite aggregate decreases much faster than the equilibrium concentration in the system of such units in contact. Therefore, the quasi-wire small aggregate shown in the previous Figure 5(a) can be considered as the basic structure and sensor unit of the film. The resistance and sensor response of the film depend on the characteristics of such units as well as on the density of contacts between the aggregates in the film [20] . These parameters therefore increase with decreasing size of aggregates and corresponding increase of their concentration in the film.
The crystallite sizes of In2O3 and ZnO, as well as the size and shape of the aggregates formed from these crystallites are determined by the deposition of the film. It should be noted that the In2O3 crystallizes in the form of cubic crystals, which can have three types of cells that differ in the mutual arrangement of the atoms and the interatomic bonds [26] . The complexity of the In2O3 lattice influences the properties of the crystals (in particular, lattice thermal conductivity [27] ) and hinders the growth of crystals during the deposition of In2O3 from aerosol. This explains the finding in a previous Figure 4 that SPT-synthesized In2O3 film comprises of splices of particles of arbitrary shape. In contrast, ZnO film consists of large-grained crystalline particles. It is also significant that in contrast to In2O3, ZnO crystallizes with a hexagonal structure [28] , such that the interaction between these oxides during the SPT-deposition of ZnO + In2O3composite film should create additional hindrance to the growth of crystals.
As a result, the rate of nucleation may be greater than the rate of crystal growth. This may cause the formation of a large number of small crystals, which combine into the aggregates. Perhaps the nature of crystallization during deposition of ZnO + In2O3composite sensor films with a high composition of In2O3 is responsible for the formation of a large number of composite aggregates of small size, shown previously in Figure 5(a). The shape of these aggregates indicates deformation of aggregates in the course of their formation, which may also be explained by the hindrance to the growth of crystals in the composite. As shown in the photomicrographs of the composite films with various ZnO to In2O3ratios (Figure 5), the composite films composed of 0.25ZnO + 0.75In2O3 besides their essential sensory properties [29] , are characterized by a homogeneous dispersive structure with high specific surface. Thus, not only the electronic structure, but also the morphological properties of the film composition 0.25ZnO + 0.75In2O3 with high density of contacts between crystal aggregates provides enhanced sensory effect in the detection of hydrogen in air ambience.
5. Conclusions
Three types of films comprising ZnO, In2O3 and ZnO + In2O3 composite were produced by spray pyrolysis technique (SPT) using water solutions of zinc chloride and indium nitrate precursor. The polycrystalline films obtained contain hexagonal wurtzite-type ZnO crystals and cubic In2O3 crystals. The study also considered the dependence of the morphology of the films on the synthesis conditions. It has been shown that increasing the deposition temperature in the range 350˚C to 450˚C results in the formation of films with more uniform particle sizes and the size of deposited particles increases with increasing concentration of precursor solution from 0.1 to 0.3 mol/liter. The particles of the film are not separate crystallites, but splices or aggregates of crystallites. The interaction between crystallites inside crystalline aggregates is stronger that the interaction between aggregates. The conductivity of the film is determined by the electron density in an aggregate and the contacts between aggregates.
The morphology of the ZnO + In2O3 composite films depends strongly on the composition of the film. It is shown that the film of composition 25 wt% ZnO + 75 wt% In2O3 contains a large number of small crystal aggregates of arbitrary shape and therefore has a high density of contacts between the aggregates. It should be emphasized that only the films with good homogeneous structure demonstrated high sensory properties. In this range of aggregate composition, the relationship between the particles of the catalytically active component (ZnO) that breaks hydrogen molecules and In2O3 particles with a high concentration of conduction electrons is close to optimal value. Therefore, due to both morphology and electronic structure of the films of this composition, the sensor response to hydrogen in air ambience is able to reach the maximal value.
Acknowledgements
The project was supported by the National Science Foundation under grant number CMMI-1030689 and the Russian Scientific Foundation grant No. 14-19-00781.
NOTES
*Corresponding author.