Synthesis and Characterization of Polyethylene Oxide Incorporated with Cadmium Sulphide Nanoparticles ()
1. Introduction
Nanoparticles are generally categorized as the class of materials that fall between the molecules and bulk materials with an average size between 1 to 50 nm [1] . Nanoparticles exhibit physical and chemical properties that are different from either the individual molecules, hence attracting an enormous attention during the past few decades. The change in the property of nanoparticles is driven mainly by two factors namely: 1) the increase in the surface area to volume ratio; and 2) change in the electronic structure due to the quantum mechanical effects with decreasing particle size [2] . The synthesis of semiconductor nanoparticles in colloidal solutions has attracted many researchers due to their unique optoelectronic properties and quantum confinement effects from the bulk materials [3] - [5] . The potential applications of semiconductors in optical switching, single charge me- mories, single electron transistors, etc. are most investigated. The physiochemical properties of semiconductors are greatly affected by the consequences of low dimensionality when the radius of the particle is comparable to the Bohr radius of the exciton versus the bulk material. Modification in the electronic levels occurred very strongly due to the limited number of atoms in the particles. Such materials in these regime exhibit novel physical and chemical properties due to the large surface to volume ratio as well as size quantization effect in semiconductor nanoparticles [6] [7] . There are several methods of forming and controlling the size of NPs in liquid phase. Capping agents and surfactants prevent uncontrolled growth and agglomeration of the nanoparticles.
Semiconductor nanoparticles have been used in hybrid solar cells [8] . In particular, cadmium sulphide (CdS) is an excellent photosensitive material and has a direct band-gap (𝐸𝑔) of 3.42 eV. The good match of its energy levels with those of semiconductors makes it a good candidate as electron acceptor coupled with polymer as electron donor to form the active layer in hybrid solar cells [9] .
There are several methods to obtain CdS nanoparticles like gas phase reaction (with H2S or sulfur vapor), solvothermal method, solution precipitation, and microwave method [10] - [12] .
For solar cell applications, however, it is very important to study how the preparation conditions affect the physicochemical properties of synthesized CdS products, and especially the photovoltaic performance of corresponding hybrid solar cells. The CdS nanoparticles (CdS-n) were synthesized by microwave assisted solution precipitation method with two different sulfur compounds: thioacetamide and thiourea. The structural and optical properties of the obtained products were analyzed and compared. It is found that the former were random distributed hexagonal particles, whereas the latter were almost monodispersed spherical ones.
Polymers that can act as coordination sites for cadmium ion aggregation have protected semiconductor nanoparticles. CdS nanoparticles covered by starch and especially amylose form a wide range of inclusion complexes. Soluble starch added during the synthesis has been used as a capping agent in the synthesis of CdS nanoparticles, resulting in well controlled and uniform particles sizes of cadmium-rich nanoparticles.
Polymer/CdS nanocomposites such as polyvinyl carbazole and CdS/polystyrene have been synthesized using various methods [13] - [15] .
The main purpose of the present work is to synthesize and analyze the characterization of the CdS nanoparticles incorporated in polyethylene oxide (PEO). Measurements of the prepared samples were done using different techniques such as: X-ray diffraction; UV-visible spectroscopy; Photoluminescence Spectroscopy (PL); and TEM. The work provides an effective route for the production of CdS nanoparticles which might be used in the fabrication of novel optical solar cell and electronic devices.
2. Experimental Work
Polyethylene oxide (PEO) (Aldrich Chemical Company Ltd., UK) of a molecular weight Mw = 90,000. Cadmium acetate (Cd(CH3COO)2·2H2O) and thiourea (CS(NH2)2) obtained from Sigma-Aldrich Company. The films were prepared by adding » of 1.2 M cadmium acetate into solution of PEO solution and continuous stirring for about 6 h at temperature » 60˚C. The solution was left until a clear one was formed indicating a complete dissolution of cadmium acetate. 2.4 M of thiourea was added with stirring about 60 min. The color of the final solution was changes from transparent to deep yellow color due to formation of nanomaterial. Three samples were named: pure PEO, 2.5 and 5 wt% obtained PEO.
The X-ray diffraction scans were carried out using Philips PW 1390 diffractometer (l = 0.1540 nm, operated at 40 kV, 10 mA) using copper target. UV- visible absorption spectra were measured in the wavelength region of 190 - 1000 nm using V-570 UV-visible, JASCO, Japan spectrophotometer. Fluorescence spectra recorded at room temperature by a JASCO FP6500 fluorescence spectrophotometer, Japan. Observations of morphology were performed using a JEM-1011 transmission electron microscope (TEM) (JEOL, Japan).
3. Results and Discussion
3.1. X-Ray Diffraction
The X-ray diffraction (XRD) analysis is very useful to investigate the structure of the polymeric materials and it enables to find out whether a material is crystalline or amorphous. Figure 1 represented X-ray spectra of pure PEO. It is clear that two main peaks at 2θ = 19˚ and 23.2˚ for pure PEO were observed.
Figure 2 shows X-ray spectra of PEO incorporated with 2.5 and 5 wt% of CdS. The main peaks at 2θ » 24.8˚, 26.7˚, 28.4˚, 44˚, and 52.1˚ are corresponding to (100), (002), (101), (110) and (112) crystalline planes of CdS [16] - [18] . These peaks can be suggested hexagonal phase of CdS with lattice constants of a = 4.14 Å and c = 6.72 Å.
The average crystalline size of CdS nanoparticle was estimated using Scherrer’s diffraction formula [19] :
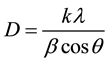
where k = 0.91, l = 0.1541 nm and b is the half-width of the diffraction. The calculated average values of the crystallite size are 49 nm and 45 nm for 2.5 and 5.0 wt%, respectively. The results indicate that the average particle size gradually decreased with increase of CdS content.
3.2. UV-Visible Spectroscopy
Figure 3 depicts UV-visible spectra of the prepared films. The onset absorption peaks around 483 nm which attributed to the transition of 1Se - 1Sh of CdS nanoparticles are observed. It is known that as far as semiconductor nanoparticles decrease to the nanosize, the energy band gap is increased. Accordingly, a blue shift took place
![]()
Figure 1. X-ray diffraction spectra of pure PEO.
![]()
Figure 2. X-ray diffraction spectra of PEO incorporated with CdS.
![]()
Figure 3. UV-visible spectra for PEO doped with CdS.
which indicates the presence of quantum confinement effect in the UV-visible absorption spectra (The bulk CdS have a peak of about 515 nm). The absorption edge is shifted towards the shorter wavelength (blue shift) with decrease in the concentration of Cd2+ ions. This indicates the formation of CdS particles in the polymeric matrix in the nanoscale. Hence, the absorption edge can be used to determine the blue shifted optical energy of the nanoparticles using the relation: Egn = hc/l, where h is the Planck’s constant and c is speed of light.
The optical band gap energy (the energy distance between the valence and conduction bands) is determined by plotting absorption coefficient α(ν) as (αhν)1/n versus hν, where n represents the nature of the transition and hν is the photon energy. Where n have values, such as 1/2, 2, 3/2 or 3 for direct, indirect, forbidden direct and forbidden indirect transitions, respectively.
The estimated values of the optical absorption spectra measurement is done using Mott and Davis concept for the direct optical band gap energy for those samples as shown in Figure 4. The band gap energy determined from the extrapolation of the linear section of the curves to x-axis in which (αhν)2 = 0 [20] [21] .
The calculated value of band gap energy decreases following the CdSconcentration from 4.04 eV to 3.32 eV for 2.5 wt% of CdS and to 2.92 eV for 5 wt% of CdS which is explained on the basis of the fact that the incorporation of amount of CdS forms charge transfer complexes in the host lattice (polymer matrix) and Cd2+ ions. This attributed to the formation of defects in polymeric matrix. These defects produce the localized states in optical band gap. These overlaps are responsible for decreasing energy band gap when CdS-filler is increased in polymeric matrix.
3.3. Photoluminescence Analysis (PL)
The photoluminescence (PL) spectra of the samples were recorded at room temperature as shown in Figure 5. It is observed that, there is no any photoluminescence emission of pure PEO and there is a broad peak at 475 nm which attributed to number of trap states. Blue shift is seen with a decrease in the intensity associate with larger size of CdS nanoparticles. This trend is due to quantum confined effect of the samples. The emission of this peak is involved the recombination of electrons trapped inside a sulfur vacancy with a hole in the valence band of the CdS nanoparticles [22] .
3.4. TEM Analysis
Figure 6 depicts TEM micrograph of morphology and distribution of CdS within PEO matrix. It is observed that more single particles which have size » 44 nm were obtained. Moreover, a higher density particles and tiny clusters with spherical shapes were connected together with uniform distribution of these spheres in PEO matrices.
4. Conclusion
The conclusion after synthesis and characterizing of cadmium sulphide (CdS) nanoparticles dispersed within polyethelene oxide (PEO) is all data from the different techniques indicate that the average particle size gradu-
![]()
Figure 4. The plots of (αhν)1/2 versus hufor PEO blend loaded by 2.5 and 5 Wt% of CdS.
![]()
Figure 5. Photoluminescence emission (PL) spectra for PEO with different content of CdS.
![]()
Figure 6. TEM micrograph of morphology and distribution of CdS within PEO matrix.
ally decreases with increase Cd salt amount. Five peaks in X-ray diffraction are observed, indicating hexagonal phase of CdS. The variation of the band gap and the particle size was attributed to the strong quantum confinement effect of CdS nanoparticles. The photoluminescence (PL) spectra show a broad character of the fluorescence that was attributed to a number of trap states. The blue shift was observed in PL attributed to association with the size of nanoparticles and the complexity of the 3D structures, indicating the quantum confined effect of the nanocomposite films. From the TEM images, the average of particle size is 44 nm and it decreases with increasing the amount of Cd, which suggests that the particle size is improved with increasing of the amount of CdS content in PEO matrices.
NOTES
*Corresponding author.