Toluidine Blue O-Gelatin Gel Dosimeter for Radiation Processing ()
1. Introduction
Ionizing radiation energy absorbed in the conventional Fricke solution [1] [2] causes oxidation of ferrous ions (Fe2+) into ferric ions (Fe3+) [3] .proposed incorporating the Fricke solution into a gel matrix to spatially stabilize the dosimetric information so that the dose distribution could potentially be measured in three dimensions. With the addition of the metal ion indicator xylenol orange (XO), Fe3+ binds to XO forming a 1:1 colored complex (XO-Fe3+) in the visible range that can be measured spectrophotometrically [4] . The irradiated-dose distribution in Fricke-type gel dosimeters has traditionally been evaluated with magnetic resonance imaging (MRI) [5] for the purpose of 3D radiotherapy dosimetry. However, as the resulting irradiated-dose distribution diffuses over time, the evaluation technique is time-limited due to the resulting blurred dose distribution. Alternative optical evaluation techniques (Laser-scanned agarose gel sections for radiation field mapping) [6] . Hydrogels can be obtained in various ways. The following methods are most widely employed: chemical cross-linking [7] mostly using glutaraldehyde as the cross-linking agent [8] ; cross-linking by γ-radiation [9] [10] , by UV radiation [11] , and by use of successive freezing/thawing cycles [12] [13] . Intermolecular bonds (mostly hydrogen bonds), which are formed during the freezing/thawing process of PVA water solutions, act as efficient cross-links [14] . Gelatin has the advantage of dissolving at a lower temperature (about 41˚C) than agarose (about 90˚C), meaning less oxygen is lost during heating that agarose is translucent, whereas gelatin is transparent, which is very important for optical absorbance measurements [15] . One chemical method, the gel dosimeter, in which substances carrying dosimetric information are suspended in a gel matrix, allows dose distributions to be measured in three dimensions (3D), promising true 3D quality assurance measurements in radiotherapy treatment planning [16] .
This paper investigates dose response characteristics of a range of gel mixture to find out the most suitable way for practical low dosimetry applications in radiation processing.
2. Experimental Work
2.1. Materials
Gelatin from porcine skin (300 bloom, G2500, Sigma-Aldrich) was dissolved in Milli-Q water and then the dye Toluidine Blue O (TBO) dye C15H16N3S+Cl−, Scheme 1 (Qualikems Fine Chemicals Pvt, Ltd., New Delhi-110 006, India), was added from a stock solution. The mixture was continuously stirred in a water bath. The solution forms a blue color. Since the reaction rate is dependent on temperature, the water bath was maintained at 70 ± 5˚C for 4 h. This temperature was chosen for a fast color change while keeping the temperature reasonably constant. Samples were pipette into 1 cm thickness glass test tube and immediately placed in a refrigerator at approximately 4˚C for 4 h. four different concentrations of dye (1.48, 3.55, 5.92 and 11.84 µmol∙L−1) and gelatin concentration is 20% w/w (that is, the mass of gelatin relative to the mass of the final gel).
2.2. Apparatus
γ irradiations were carried out with a Gamma chamber 4000A 60Co irradiation facility (BARC, India). The absorbed dose rate in the irradiation facility was measured to be 1.37 kGy∙h−1. Uvikon 860 spectrophotometer (KONTRON Co. Ltd., Switzerland) was used to measure the absorption spectra of the unirradiated and irradiated samples.
3. Results and Discussion
3.1. Absorption Spectra
The absorption spectra of the un-irradiated and irradiated shows absorption band in the visible region peaking at 635 nm (characteristic to a blue color) for dyed polymer gel (Figure 1). It is shown that the amplitude of all absorption bands in the visible spectra decrease gradually with increase of the dose of gamma-ray photons.
3.2. Dose Response
Group of gels were irradiated with dose rate of 1.37 kGy/h in the range between 1 and 150 Gy. Over that dose, the response tends to saturate. The resulting gel color has an absorbance peak at 635 nm, this peak bleaches upon irradiation as the gel bleaches. Figure 2 shows dose response functions of the gel-dyed samples with various concentrations OTB. Each dose point corresponds to four replicate test tube samples. The dose dependences are linear up to 30 Gy. Figure 3 show the linear correlation coefficients were found to be 0.0215, 0.025678, 0.033918 and 0.03921 for the prepared mixture (gel-dyed) concentrations 1.48, 3.55, 5.92 and 11.84 µmol∙L−1, respectively. The sensitivity of the gel samples to radiation doses, expressed as the slope of the dose response curve, increases linearly with the dye concentration in Figure 4, the gel samples with 11.84 µmol∙L−1 of the dye content are about 4 times more sensitive than the film with 1.48 µmol∙L−1 of the same mixture.
3.3. Radiation Chemical Yield (G-Value)
The radiation chemical yield can be expressed as the number of moles of the dye degraded by absorption of 1 J of energy. The G-value was calculated using the general relation:
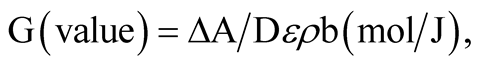

Scheme 1. The absorption spectra of (TBO-gelatin) gel unirradiated and irradiated to different absorbed doses.

Figure 1. The absorption spectra of (TBO-gelatin) gel unirradiated and irradiated to different absorbed doses.

Figure 2. Dose response of the (TBO-gelatin) gel at 635nm in the full dose range of 1 - 150 Gy.
where ΔA is the change in absorbance at λmax, b is the optical path length (1 cm), ε is the linear molar extinction coefficient for the solution at λmax (L∙mol−1∙cm−1), ρ is the density of the polymer gel (g∙cm−3), and D is the absorbed dose (Gy). The molar extinction coefficient of TBO had been found to be 1.740049 × 105 L∙mol−1∙cm−1. The radiation chemical yield was calculated from the linear portion of the response curve (ΔA vs. dose). Figure 5 shows the calculated G-values for various dye concentrations.
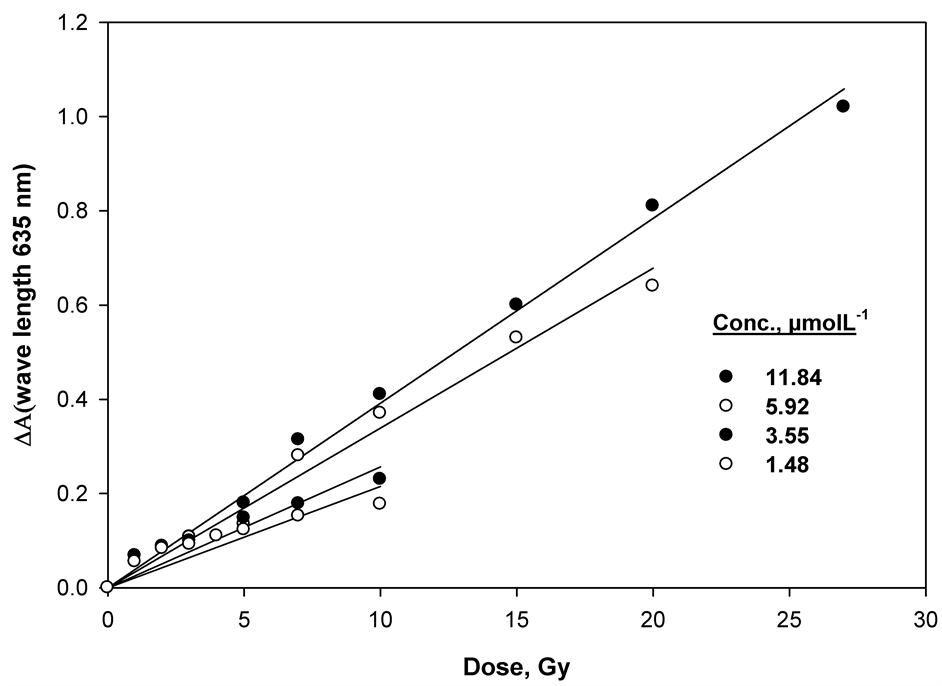
Figure 3. Linear dose response of the (TBO-gelatin) gel at 635 nm in the low-dose range of 1 - 30 Gy.

Figure 4. Change of radiation sensitivity of TBO containing gel mixture as a function of concentration of dye.

Figure 5. Change of G-values at 635 nm as a function of concentration of (TBO) dye.
3.4. Pre-Irradiation Stability
To investigate possible effects of pre-irradiation storage on the manufactured gel samples, we monitored absorbances of unirradiated gel samples stored under different conditions. Two groups of gel samples manufactured approximately one month before the start of the experiment we restored under different conditions, and their absorbances at 635 nm we monitored for 36 days. One of the groups was stored at room temperature in the dark; another group was stored at room temperature exposed to laboratory fluorescent light Figure 6, the absorbances of the samples stored in the dark at −4˚C remained essentially unchanged during the whole period of the observations. The absorbances of the samples stored at room temperature in the dark changed about 3% over the same period of time. However, the absorbances of the films stored at room temperature under fluorescent light increased approximately 8% by the end of the observation. So; storage of unirradiated films in the dark at −4˚C is recommended.
3.5. Post -Irradiation Stability
Multiple (TBO-Gelatin) gels were irradiated to 15 Gy approximately one month after their manufacturing. After the irradiation, they were stored under different conditions. One group was stored at room temperature under laboratory fluorescence light; and another group was stored at −4˚C in the dark. The absorbances of the samples at 635 nm were measured periodically over 36 days of storage Figure 7. The signals of the samples stored at −4˚C were very stable over the whole observation period. On the contrary, the responses of the samples stored at room temperature under laboratory fluorescence light, increased rapidly during the first week of storage and then grew more slowly until the end of the observation period.

Figure 6. Pre -irradiation stability of (TBO – gelatin) gels stored under different storage conditions

Figure 7. Post -irradiation stability of (TBO – gelatin) gels stored under different storage conditions.
4. Conclusion
We evaluated the high-radiation-sensitive polymer gel as a possible 3D dosimeter. The polymer gel had highly accurate radiation sensitivity in the dose range of 1 - 150 Gy, and calculated dose distributions were in good agreement with the gel measurements. The polymer gel can be produced in various shapes for specific applications; this can be done with easy quality control and at low cost. These characteristics are suitable for the verification of the relative dose distribution in clinical uses. The spectrum of the prepared dyed gel samples undergoes a change upon gamma irradiation, and bleaching of the blue gel color increases with the radiation dose; they are suitable for use in monitoring various radiation-processing applications, which can be useful for most of food irradiation applications (seed production and inhibition of sprouting), insect population control, blood irradiation and medical radiotherapy. The response of the gel-dyed mixture was not affected by variations of environmental conditions, and the prepared gel samples show an excellent stability before and after irradiation except the first 5 days.