n-Hexadecanoic Acid

1. Introduction
Man could land on Mars but failing to outwit a tiny creature i.e. mosquito over centuries. Due to imprudent land use pattern by man, concurrence on mosquito becomes a challenging task. Mosquito menace had been a manifold problem for years together in tropical and subtropical regions. The root cause for mosquito density and its causative disease burden is obviously because of the nature of mosquitoes’ complex life cycle which is tuned to adapt successfully to diversified habitats. Despite the time-tested mosquito control efforts, there are still mosquito-borne diseases bothering the people across the world and especially in India.
The city like Hyderabad in India is severely suffering from the annoyance of mosquitoes, which even ruins the pleasure of patio. To pacify the panic of the people who are scientifically illiterate, civic facility providers unfortunately do fogging in human habitations on regular basis. Control of mosquitoes using adulticides is not a prudent strategy, as the adult stage occurs alongside human habitation, and they can easily escape from remedial measures [1] . More so, most of the insecticides available in the market are synthetic chemical products apart from their prohibitively high-cost persistent applications which have unintended implications including the production of resistant strains of mosquitoes, ecological imbalance and elimination of non-target organisms in the environment [2] .
It is imperative that the effective anti-larval measures can reduce the burden of mosquito menace to a greater extent. And applications of such anti-larval measures with the extracts or essential oils from plants are potential alternatives for mosquito larval control, as they constitute a rich source of bioactive compounds that are biodegradable into non-toxic products and potentially suitable for use in control of mosquito larvae. In fact, many researchers have reported on the effectiveness of plant extracts or essential oils against mosquito larvae [3] -[5] . But, its application in the field is yet to be ascertained. However, biological reduction of metal would be boon for the development of clean, nontoxic, and environmentally acceptable metal nanoparticles; the formed silver nanoparticles are hydrophilic in nature, disperse uniformly in water, highly stable, and had significant mosquito larvicidal activity [6] .
Nanoparticle synthesis is the widely growing area of nanotechnology. In recent times synthesis of nanoparticles has been a promising field particularly in medical applications [7] . Nanoparticles of noble metals are found to have potential applications in various fields like electrochemical deposition [8] . Synthesis is of nanoparticles using biological entities having great interests due to their unusual optical [9] , photo electrochemical [10] and drug delivery system [11] .
Thus, researchers are exploring to develop novel materials as mosquito larvicide. Metal nanoparticles are emerging as one of the fastest growing materials due to their unique physical, chemical and biological properties [12] . The peculiar properties of silver nanoparticle are obviously small in size, high surface area, easy to suspend in liquids and deep access to cells and organelles that can easily strike on the mosquito larvae in the aquatic phase of its life cycle. It was reported to have amino acids which are proposed to play a key role for the reduction of silver ions and the formation of corresponding nanoparticles [13] . Currently, there is a growing need to develop environmentally benevolent nanoparticle synthesis that does not use toxic chemicals in the synthesis protocols [14] . Biosynthesis of silver nanoparticles using plants, bacteria, fungi and yeast [15] -[18] is known to reduce silver ions into silver nanoparticles by both extra and intra cellular level. Reports are available on larvicidal activity against various mosquitoes [19] [20] . In the present study an attempt was made to explore the larvicidal activity of silver nanoparticles synthesized using dried leaf extract of P. pinnata.
2. Material and Methods
2.1. Plant Material
Pongamia pinnata (L) Pierre (Fabaceae family) plant leaves was collected from Vikarabad forest, Rangareddy district, Andhra Pradesh, India (17˚20'0"N, 77˚54'0"East). Pongamia pinnata (Fabaceae family) was taxonomically identified by Prof. P. Ramachandra Reddy, Taxonomist, Department of Botany, Osmania University, Hyderabad, India. A voucher specimen was deposited in the Department of Zoology, Osmania University.
2.2. Processing of Plant Material
The leaves of Pongamia pinnata was shade dried at room temperature, for 10 - 15 days. The dried plant material was grinded to powder with an electrical stainless steel blender. The powder was socked with ethanol (99.5%) solvent and extracted using Soxhlet apparatus for 8 hrs. The extract was concentrated to paste with rotary evaporator at 65˚C for 4 hours and the residue obtained was stored at 4˚C in an airtight bottle until further use.
2.3. Preparation of Stock Solution of Plant Extracts
1 gm of extract was taken and dissolved in 1000 ml of double distilled water and filtered using Whatman filter paper No 1. It was stored at room temperature for further experiments as stock solution (1000 ppm).
2.4. Synthesis of Silver Nanoparticles (AgNPs)
In a typical synthesis of silver (Ag) nanoparticles, the leaf extract (1.0 ml) was added to 20 ml of 10−3 M AgNO3 (99.99%) aqueous solution and kept at room temperature. The experiment was done in triplicate for reproducibility. After 1 hour the colour of the solution changed from colourless to honey brown indicating the formation of silver nanoparticles and this is confirmed by UV-visible spectroscopy.
2.5. Characterization of AgNPs
The bio reduction of pure Ag+ ions was monitored by measuring the UV-visible spectra of the reaction medium. UV-visible spectral analysis was carried out with a SHIMAZDU 2600-(TCC) UV-visible absorption spectrophotometer with a resolution of 1 nm between 200 and 700 nm. A small aliquot of 300 µL of the sample is diluted 10 times with Millipore water to avoid errors due to high optical density of the solution.
The crystalline nature of the nanoparticles was measured using X-Ray Diffractometer (XRD) by depositing thin film of the silver nanoparticles was made by dipping a glass plate in the solution and carried out the X-ray studies.
For FTIR measurements, the silver nanoparticles solution was centrifuged at 10,000 rpm for 30 min. The pellet was washed three times with 20 ml of de-ionized water to get rid of the free proteins /enzymes that are not capping the silver nanoparticles. The samples were dried and grinded with KBr pellets and analysed on a Bruker Optics (Germany made) Tensor 27 model in the diffuse reflectance mode operating at a resolution of 0.4 cm−1.
The size and shape of silver nanoparticles are visualized through the 200 kV Ultra High Resolution Transmission Electron Microscope (JEOL-2010). TEM grids are prepared by placing a drop of the particle solution on a carbon-coated copper grid and drying under lamp.
2.6. GC-MS Analysis
GC-MS analysis was done by the SHIMAZDU QP2010, an oven temperature from 50˚C to 280˚C at 4˚C/min and held at this temperature for 5 min; inlet and interface temperatures were 250˚C and 280˚C, respectively. Carrier gas was He at a flow rate of 1.0 ml/min (constant flow). 0.2 ml of sample was injected under split of 20:1. EIMS: electron energy, 70 eV.
2.7. Identification of Compounds
Interpretation of mass spectrum GC-MS was conducted using data base of NIST, having more than 62,000 patterns. The spectrum of the known compounds was compared with the NIST library.
2.8. Dose-Preparation
Based on the preliminary screening results the leaf extract of Pongamia pinnata and synthesized silver nanoparticles were subjected to dose-response bio-assay for larvicidal activity of Ae. albopictus larvae. The synthesis of leaf mediated silver nanoparticle of P. pinnata was estimated at a concentration of 0.25 ppm (Lc50) and 1ppm (Lc90). Whereas in solvent extraction with ethanol was estimated at a concentration of 100 ppm, 150 ppm, 200 ppm and 250 ppm [21] .
2.9. Bioassay
According to WHO (2005) bioassay test was performed with different concentration to assess the larvicidal activity [22] .
2.10. Statistical Analysis
The average larval mortality data were subjected to Probit analysis (FORTRAN) for calculating Lc50 and Lc90 [23] .
3. Results
Pongamia pinnata leaf solvent (ethanol) extract was subjected to synthesis of silver nanoparticles and the visible color change (Figure 1) indicates the formation of nanoparticles which is confirmed by UV-visible absorption spectroscopy. The progress of the reaction between metal ions and the leaf extracts were monitored by UV-visible spectra of silver nanoparticles in aqueous solution with different reaction times that are shown in Figure 2. It was observed from the figure that the peak blue shifted in the absorption spectrum from 437 nm to

Figure 1. Shows the photograph (A—leaf extract, B—silver nanoparticles after 1 hour) of leaf extract of Pongamia pinnata.
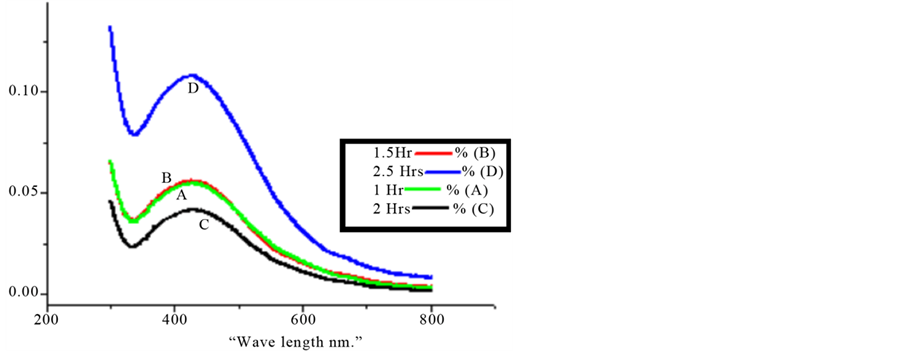
Figure 2. UV-visible spectra recorded as a function of time of reaction of an aqueous solution of 10−3 M AgNo3 with the leaf extract of Pongamia pinnata. (a) 30 minutes; (b) 60 minutes; (c) 90 minutes; (d) 120 minutes.
420 nm with increasing reaction time from 30 min to 120 min. It took two hours to complete the reaction to form stable nanoparticles.
The crystalline nature of silver nanoparticles was confirmed by the X-ray diffraction analysis. Figure 3 shows the XRD pattern with the diffraction peaks at 44.50, 52.20 and 76.7 corresponding to the (111), (200) and (220) facets of the face centred cubic (FCC) crystal structure. The broadening of the Bragg peaks indicates the formation of nanoparticles. In addition to the Bragg peaks representative of FCC silver Nano crystals, additional, and yet unassigned, peaks were also observed suggesting that the crystallization of bio-organic phase occurs on the surface of the silver nanoparticles.
FTIR spectroscopy analysis was carried out to identify the potential bio molecules in the leaf extract responsible for the reduction and also the capping reagent responsible for the stability of the bio reduced silver nanoparticles. A typical FTIR spectrum of the obtained silver nanoparticles is shown in Figure 4, the absorption bands at 3359, 3018, 2574, 2133, 1738, 1642, 1438, 1366, 1218 and 1100 cm−1. The intense band at 3359 is corresponds to O-H stretching, 3018 is =C-H Medium stretching, 2574 Carboxylic acid O-H stretching, 2133 is corresponding to C-H stretching, 1738 is due to stretch vibration of -C=O, the band at 1642 is corresponds to amide I, arising due to carbonyl stretch in proteins. The bands at 1366 and 1218 correspond to C-O stretch (phenolic) and ester phenolic compound respectively. The weak band at 1100 is -C-Ostretch. It is observed from the spectra of silver nanoparticles the appeared bands at 1642 and 3359 which are due to hydroxyl group and amide-I that are responsible for reducing the Ag+ ions to atoms and suppressed bands at 1738, 1366 and 1218 are responsible for stabilising the nanoparticles.
TEM technique was employed to visualize the size and shape of silver nanoparticles. TEM grids were prepared by placing a drop of the particle solution on a carbon coated copper grid and dried under lamp. Figure 5 shows the typical bright-field TEM micrograph of synthesized by reduction of Ag+ ions with 1 g biomass are predominantly are of the spherical shape. It is evident that there is variation in particles sizes and average size estimated was 20 nm and the particles size ranged from 10 nm to 80 nm and above Figure 5 corresponds to high resolution lattice image form one such particle.
The chromatogram of Gas Chromatography-Mass Spectrometry (GC-MS) shows that the active ingredients in synthesized silver nanoparticles of the leaf extraction (Table 1) was analyzed and found that there were approximately 55 active compounds recorded within 25 minutes of retention time. However, there were two prominent compounds found at different peaks in chromatogram are (at peak number 9 and 15) namely

Figure 3. XRD pattern of as synthesized silver nanoparticles of Pongamia pinnata leaf extract.

Figure 4. FT-IR spectrum of AgNPs prepared from the Pongamia pinnata plant extract.

Figure 5. TEM image of bio-reduced silver nanoparticles.
9-Octodecenoic acid (Z) (C18 H34 O2) and n-hexadecanoic acid (C16 H32 O2) with a maximum peak area i.e. 34.47% and 3.01% respectively and both are having larvicidal property [24] [25] . Therefore, it is derived that 9-Octodecenoic acid and n-hexadecanoic acid are responsible as potential larvicides at Nano scale which are environmentally benign.
4. Discussion
Pongamia pinnata has been regarded as medicinally important plant and earth-friendly herbal pesticide. The efficacy of larvicidal activity of this plant was reported at 118.2 ppm (Lc50) and 227 ppm (Lc90) by Raut et al. (2010) [26] [27] . However in our study we found that Lc90 was recorded at 100 ppm in solvent (ethanol) extraction and the AgNPs of P. pinnata has shown the significantly lower concentration in comparison to earlier publications of their


Table 1. GC-MS Analysis of Pongamia pinnata leaf extract.

Table 2. Larvicidal activity of the Pongamia pinnata leaf extract and synthesis of silver nanoparticles against Aedes aegypti.
larvicidal property i.e. at 0.25 ppm (Lc50) and 1.0 ppm (Lc90). The results of upper confidential level (UCL), lower confidential level (LCL) were mentioned in Table 2. According to Raut et al., the presence of prenylated flavonoid derivatives like pongaflovinol and tunicatachalcone in P. pinnata was reported, where the later one is proposed as biogenetic precursor of the former one and the reaction is effected by flavanone/dihydroflavanol NADPH-dependent reductage. Therefore, it reflects the compounds, such as 9-Octadecenoic acid (Z) and nhexadecanoic acid, which are reducing and capping ligands of the nanoparticles responsible for the larvicidal activity.
Acknowledgements
The work was supported by (F. No. 40-417/2011(SR); Dt.4-7-2011) University Grants Commission New Delhi.
NOTES
*Corresponding author.