Radiation Induced Grafting of Acrylic Acid onto Viscose Rayon Fabrics and Its After-Effects ()
Received 5 July 2016; accepted 28 August 2016; published 31 August 2016

1. Introduction
Radiation induced graft copolymerization of vinyl monomers onto various polymeric materials or textile fabrics proved to be a simple and effective means for modifying the chemical and physical properties of various polymeric substrates [1] - [3] . For example, radiation induced graft copolymerization of acrylic acid onto Nylon-6 gave fibers with better dyeability [4] . Grafting of vinyl pyrolidone onto Nylon-6 by gamma radiations has been reported to result in modified fibers with higher moisture regain and tensile properties [5] . Moreover, the wet crease recovery of viscose rayon has been found to improve by grafting with acrylic acid monomer [6] . For better control over the nature of changes occurring in grafted products, in some cases mixed monomers have been applied. Thus, graft copolymers prepared from polypropylene films with mixed monomers e.g. acrylic acid together with styrene, were reported [7] .
Viscose rayon is a rather popular synthetic fabric. It would be very useful to investigate the possibility of modifying its physical properties to increase its value as an industrial technical product. Perusal of literature showed that very little data are available in that respect.
In the present work, samples of viscose rayon fabric were grafted with acrylic acid monomer by gamma radiations under different experimental parameters including solvent type and composition, irradiation dose, etc., in a trial to observe the changes in the properties of the modified fabrics, particularly the dyeing properties, as a result of the applied treatments.
2. Materials and Experimental Techniques
2.1. Materials
2.1.1. Viscose Rayon
Untreated pure viscose rayon fabrics were obtained from Misr Helwan company, Helwan, Egypt. Before being used viscose rayon fabrics (in the form of 7 cm width stripes) were extracted for 30 minutes with diethyl ether then with ethyl alcohol in a Soxhlet apparatus and finally were washed with distilled water. The samples were dried in a vacuum oven at 60˚C until constant weight. The dried samples were stored in a vacuum descicator until used.
2.1.2. Monomers
Acrylic acid was obtained from Prolabo, Rhone, France. It was purified by distillation at 48.5˚C under reduced pressure (15 mm∙Hg) in the presence of 5% hydroquinone as stabilizer.
2.1.3. Solvents
Double distilled water was used all over the work. Analar methanol was obtained from Prolabo, Rhone, France.
Dimethyl formamide (DMF), a pure grade product, was obtained from Veb-Laborchemie, Germany and was used without further purification.
Analar benzene was obtained from the B. D. H., GB and used without further purification.
2.1.4. Solid Inorganic Chemicals
A. R. Ferrous ammonium sulphate [(NH4)2∙SO4∙FeSO4∙6H2O], Nickel (II) chloride [NiCl2∙6H2O] and cobalt (II) sulfate [CoSO4∙7H2O] were obtained from B. D. H, GB.
2.1.5. Dyes
The following dye types were used in the present work. The direct dye 23, Diamine Fast Scarlet 485, C. I. 2916 and the disperse dye Celliton pink FF3, were obtained from BASF, Germany. The basic dye Sandocryl blue B-3 Gr, C. I. 41004 was obtained from Sandoz Germany.
2.2. Preparation of Samples for Irradiation
In the present work, irradiation grafting of viscose rayon fabrics with acrylic acid monomer under different experimental parameters was studied.
For irradiation of samples, pyrex glass tubes were used, 2 cm in diameter and 15 cm long, provided with two break seals, one for closing the tubes before irradiation and the other for opening them after irradiation.
In all experiments, exactly about 0.5 g of viscose rayon samples were introduced into the glass irradiation tubes. Fourty ml of the liquid phase, consisting of methanol/water or Dimethyl formamide/water, containing a certain amount of acrylic acid monomer and 25 micromoles of Fe2+ ions, were finally added taking care that the contents in the irradiation tubes did not exceed two thirds of their total volume. The contents in the tubes were deaerated by bubbling Ar or N2 then were freezed, sealed and introduced into the gamma cell for irradiation.
2.3. Gamma Irradiation
A Cobalt-60 Canadian gamma cell 220 of the National Centre For Radiation Research And Technology, Nasr City, Cairo with a dose rate 1.8 kGys/hour was used. Some preliminary studies were carried out using the gamma cell facility placed in the Nuclear Research Center, Physics Department, Inshas, Cairo, with a dose rate 280 grey/hour. The irradiated samples were arranged in a carton rack inside the irradiation volume in order to specify the isodose positions in the irradiation volume inside the cell.
2.4. Treatment of Irradiated Samples and Determination of the Graft Yield
At the end of the irradiation period, the tubes were cooled then opened within one hour after their removal from the irradiation field. In those cases where post-irradiation heat treatment or storage of the sealed irradiation tubes was applied, the tubes were opened after the appropriate treatment and the grafted viscose rayon samples were removed, thoroughly washed with hot water, extracted with water in a soxhlet apparatus for three hours then carefully dried and finally weighed. The extraction process was repeated until the grafted samples acquired a constant weight.
The graft yield was then calculated by the equation:
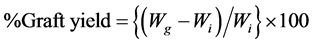 ;
;
where Wi is the initial weight of the viscose rayon sample used and Wg is the weight of the grafted viscose rayon sample after washing and drying.
3. Investigation of the Properties of the Grafted Viscose Rayon Fabrics
3.1. Determination of Moisture Absorption (%)
The grafted viscose rayon sample was placed in a weighing bottle then dried in a vacuum oven at 100˚C - 110˚C for two hours to ensure the complete removal of moisture, then kept to cool in a desiccator. The weight of the dry sample, Wd, was determined. The weighing bottle containing the dry sample was then kept for 24 hours in a desiccator containing saturated sodium nitrite solution to give 65% relative humidity at 21˚C. The weight of the wet grafted sample was then determined, Ww. The percent moisture absorption was then calculated from the following relationship:
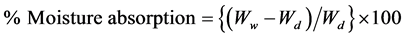
3.2. Determination of Swelling (%)
The grafted viscose rayon sample was placed in a weighing bottle, completely dried in a vacuum oven then kept to cool in a desiccator and weighed. That process was repeated until constant weight, Wd. The dry sample was immersed in distilled water for 24 hours then centrifuged for 5 min. at 2000 r/min, carefully removed and finally weighed, Ww.
The percent swelling was then calculated as follows
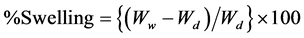
3.3. Determination of the Dry Crease Recovery
The dry crease recovery angle of the grafted samples was determined using a special Hungarian apparatus type FF-07. Measurements were carried out according to the standard ASTM method twice, on the weft and warp directions of the grafted samples. A load was applied for 15 minutes then the load was released and the sample was left free for 15 minutes and the formed angle was finally measured.
3.4. Determination of Tensile Strength and Elongation at Break
The untreated and grafted samples used for tensile strength and elongation at break measurements were tested according to ASTM638 specifications. An Instron Universal tester (GB), was used throughout the present work by applying a crosshead speed of 10 mm/min at room temperature with tension and compression capacity of 10 tons. Squared samples were prepared with a side length of 5 cm and placed between the jaws of the apparatus. Elongation at break and tensile strength were calculated according to the following:
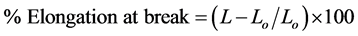 ,
,
where Lo is the initial length of the sample between the jaws, L the length after elongation. The stress in Kg/cm2 was calculated using the equation:
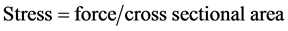
Force was calculated from the resultant chart. The cross sectional area was calculated from the following relationship
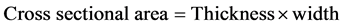
Thickness was measured using a thickness gauge micrometer.
3.5. Determination of the Dye Uptake by the Grafted Samples
In the present work, viscose rayon grafted fabric samples were dyed with three types of dyes, a direct dye, a disperse dye and a basic dye. The dyeing procedures used were the standard procedures usually applied for dyeing fabrics with these dyes.
The dye uptake in each case was determined by measuring the color strength of the produced fabrics. Thus, reflectance measurements of the dyed fabrics were carried out using 2 Hunter Lab calorimeter model P-25-M-2. The Kubelka-Munk equation [8] was used to calculate the K/S values as follows:
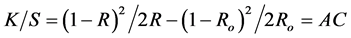
where:
R is the decimal fraction of the reflectance of the colored fabric,
Ro is the decimal fraction of the reflectance of uncolored fabric,
K is the absorption coefficient; S is the scattering coefficient,
C is the dye concentration; A is a proportionality constant.
4. Determination of the Acrylic Acid Content in the Liquid Phase Samples after Gamma Irradiation
The percent of acrylic acid remaining in the irradiation tubes containing the grafting mixture was determined by taking a certain volume of the liquid phase, adding a known excess of standard sodium carbonate solution and at the end of the neutralization reaction, the excess of sodium carbonate was determined by a standard mineral acid solution using methyl orange indicator. From the results it is possible to calculate the amount of acrylic acid remaining after irradiation and also the % conversion of the monomer. In order to test the validity of the procedure authentic samples were prepared consisting of varying amounts of acrylic acid i.e. 100 - 400 mg in 10 ml water mixed with 90 ml of methanol/water solvent mixture (7:2). The resultant samples were analysed to determine their acrylic acid content as described. The results showed that the percent recovery of acrylic acid in the authentic samples were around 100%.
These results showed that it is possible to determine the percent conversion of acrylic acid in the liquid phase used in the grafting experiments by the described procedure.
5. Results and Discussions
Grafting is a highly attractive subject in polymer chemistry since it could lead to the synthesis of new products that acquire desirable physical or technical properties. As a result of the unselective absorption of gamma radiation in matter it is possible to combine any monomer with any polymer just by applying the appropriate irradiation conditions [9] .
In the present work, radiation induced grafting of acrylic acid monomer onto viscose rayon fabric, has been studied in detail.
5.1. Factors Affecting the Grafting Yields of Acrylic Acid onto Viscose Rayon
1) Effect of acrylic acid monomer concentration in different solvent systems
The change of the graft yield of acrylic acid monomer onto viscose rayon fabric at different monomer concentrations, in two liquid solvents are shown in Figure 1. It is clear that on using methanol/water solvent mixture, the graft yield increased on increasing acrylic acid concentration up to 10% and then continues to increase, but at a lower rate up to 20% monomer concentration.
In case of DMF-water solvent system, the graft yield (%) was markedly lower than in case of methanol-water solvent system within the monomer concentration range 5% - 20% in the liquid phase. That lower graft yield could be mainly due to the decreased swelling of the viscose rayon fibers in DMF-water solvent which is accompanied with greater homopolymer formation than in case of using methanol-water solvent.
In fact on using monomer concentrations greater than 20% in both solvents excessive gelling occurred due to enhanced homopolymer formation which was difficult to remove efficiently.
2) Effect of solvent type and solvent composition
Solvents play an important role in radiation induced grafting of monomer molecules on to the active sites formed in the solid polymer matrix, by gamma irradiation. These active sites interact readily with the approaching monomer molecules from the bulk solution by diffusion. The diffusion process is enhanced mainly by the swelling of treated fabrics under the effect of the existing solvent system. In the present work, methanol-water and DMF-water solvent systems were used.
The change of the acrylic acid graft yield (%) on using different solvent systems with variable compositions, at different irradiation doses, are shown in Figure 2(A) and Figure 2(B). It is possible to observe that the grafting yield of acrylic acid onto viscose rayon increases on increasing the methanol concentration in solvent up to 80%and then decreased.
On using DMF as the solvent system the graft yields were found to decrease on increasing DMF up to 25% then remained almost unchanged until the concentration of DMF amounted to 90%, on using two different irradiation doses. That behavior could be attributed to differences in the ability of those two solvent systems to swell the fibers and probably shows that methanol/water solvent system is more capable of swelling viscose rayon fibers than DMF/water system.
3) Effect of Irradiation dose
![]()
Figure 1. Effect of monomer concentration on grafting acrylic acid onto viscose rayon fabric using different solvent systems containing 25 micromoles of Fe2+: a―Methanol-water mixture (7:2); b―Dimethyl formaide-water mixture (6:3). Irradiation dose: 20 kGy.
![]()
Figure 2. Effect of solvent composition on the graft yield of acrylic acid onto viscose rayon fabrics. Monomer concentration in the solvent used, 10%. Irradiation dose: a―20 kGy. b―10 kGy. Solvents systems, containing 25 micro moles of Fe2+: (A) Methanol-water solvent; (B) DMF-water solvent.
Viscose rayon samples were irradiated in different liquid phases containing 10% acrylic acid and 90% of each of two solvent systems, namely Methanol-water (7:2) and DMF-water (1:8) in presence of 25 micromoles of Fe2+ ions. The results are shown in Figure 3 from which it could be observed that the grafting yields did not significantly increase on increasing the gamma irradiation doses from 10 kGy up to 40 kGys. It is expected that the number of graft centers, formed on vicose rayon depends on the size of the irradiation dose. At higher doses more grafting centers are formed in and on the irradiated fiber. In presence of solvents that do not favor swelling of the fibers to promote the grafting reaction, homopolymer formation is favored. In other words, homopolymer formation increased while the grafting reaction did not increase on increasing the irradiation dose. In the present work, the graft yield increased from 10.5% to 12% on using Methanol-water solvent system and from 5.5% to 7% on using DMF-water solvent, on increasing the irradiation dose from 10 to 40 kGy. In general, homopolymer formation was markedly observed, particularly on using DMF/water solvent when doses greater than 30 kGy were applied.
4) Effect of presence of air or argon on the graft yield
![]()
Figure 3. Effect of the γ-irradiation dose on the grafting yield of acrylic acid onto viscose rayon in different solvent systems: Solvents used: a―Methanol-water (7:2); b―DMF-water (1:8). Monomer concentration: 10%.
The percent grafting of acrylic acid onto viscose rayon fabrics has been determined in two irradiated samples with methanol-water and DMF-water solvent systems. The irradiation tubes were flushed with Argon or air before sealing. It has been found that very slight differences were observed in the grafting yields recorded in both cases.
5) Effect of polyvalent metallic ions on the grafting yield at different irradiation doses
The effect of redox systems involving the use of Fe2+ ions during radiation induced grafting of polymers with vinyl monomers has been reported by many authors to reduce homopolymer formation [10] - [12] .
In the present work, a trial has been carried out to investigate the effect of other multivalent metal ions e. g. Co2+ or Ni2+ ions on the grafting yields obtained. Thus, the graft yield obtained on using increasing micromolar amounts of Fe2+, Co2+ or Ni2+ ions to the irradiated samples, are shown in Figure 4. It could be observed that, in presence of Co2+ ions the maximum percent grafting was 1.8 times greater than the corresponding values obtained in the absence of these ions. In presence of Ni2+ and Fe2+ the maximum grafting yields amounted respectively to 1.46 and 1.29 times greater than the corresponding grafting yields obtained in the absence of these ions.
It has been reported that irradiation of polymer molecules in presence of air leads to the formation diperoxides and hydroperoxides in addition to other products. In presence of Fe2+ these dissociate to give active free radicals:
 (1)
(1)
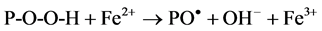 (2)
(2)
The grafting reaction occurs by diffusion of monomer molecules to the active centers PO. formed in the polymer structure
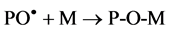 (3)
(3)
In presence of Fe2+ ions the following reaction can compete for 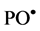 as follows
as follows
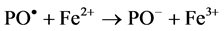 (4)
(4)
Therefore, in presence of high redox ions concentration, lower grafting yields will be obtained.
The higher grafting yields obtained in presence of micromole amounts of Co2+ ions, as compared to the yields obtained on using Fe2+ ions, could be understood by referring to the standard reduction potentials of Co2+ (+1.82 v) and Fe2+ (+0.77 v) which shows that ferrous ions are stronger reducing agents than cobalt ions. Therefore, the grafting reaction (3) is more favored in presence of Co2+ ions while in presence of Fe2+ ions reaction (4) is more favored.
![]()
Figure 4. Effect of polyvalent metal ions on the grafting yield of acrylic acid onto viscose rayon. Irradiation dose 20 kGy. Solvent system used: 10% Acrylic acid, 90% Methanol: H2O (7:2) containing increasing micromolar amounts of: a―Co2+ ions; b―Ni2+ ions; c―Fe2+ ions.
Under these conditions termination of the grafting reaction can occur as a result of Fe3+ accumulation [13] as follows:
![]()
Obviously Co3+ ions accumulate in the irradiated systems relatively to a lower extent than Fe3+ and consequently the termination reaction in case of cobalt ions occurs at a lower yield.
6) Effect of storage time of the grafted samples after irradiation
After irradiation the sealed tubes containing viscose rayon fabric samples, monomer and solvent were kept at room temperature for increasing time periods. The tubes were then opened and the grafted samples were, repeatedly washed with hot water, dried and weighed until constant weight. The grafting yields were calculated The results obtained are shown in Figure 5. It is clear that on using methanol-water solvent system, the initial percent grafting, amounting to 14%, decreased gradually to about 3%, at 28 hours after stopping irradiation. As the storage time was further increased the percent grafting was found to increase gradually to 22% when the irradiated samples were kept for 120 hours after stopping irradiation.
It is very probable that the observed change in the grafting yield during the first 28 hours after irradiation is the result of two processes viz. a decay process of the original graft copolymer A formed during irradiation and which decays completely in 28 hours after stopping irradiation and the graft copolymer B that continue formation even after the complete decay of the copolymer A. The decay curve of copolymer A, shown by line A in Figure 5, is determined by finding the difference between the decay line (A + B) representing the decay of the existing graft copolymer after stopping irradiation and the extrapolated line B representing the increase of the percent yield of copolymer B during its formation, after stopping irradiation.
Therefore, it is possible to conclude that two copolymers A and B coexist for about 28 hours after irradiation and after decay of copolymer A copolymer B continues formation. Such a behavior could be understood if we refer to the nature of the reactive free radical sites formed on the solid viscose rayon matrix upon gamma irradiation.
Viscose rayon consists mainly of cellulose macromolecules (94%) in the form of long chains containing crystalline, partly crystalline and amorphous regions [14] . The free radicals formed in the amorphous region are readily accessible to the monomer molecules or radicals formed during irradiation. It seems that their interaction is probably reversible as could be observed from the results shown in Figure 5. On the other hand, radicals formed in the ordered matrix of the crystalline regions in viscose rayon fibers are more firmly trapped due to the
![]()
Figure 5. Change of the percent graft of acrylic acid onto viscose rayon with time of storage of samples at room temperature after irradiation. The liquid phase consists of 10% acrylic acid, 90% methanol water mixture (7:2) or DMF-water mixture (6:3) and 25 micromoles of Fe2+. Irradiation dose: 20 kGy. A + B: Decay curve of the graft, formed during irradiation of viscose rayon fabric in presence of a liquid phase consisting of 10% acrylic acid and 90% methanol/water solvent mixture, after stopping irradiation. A―Decay Curve of grafted polymer A initially formed during gamma irradiation with the time of storage after irradiation. C―Decay curve of the grafted viscose rayon with acrylic acid in liquid phase consisting of 10% acrylic acid, 90% DMF-water mixture (6:3).
lower mobility of the polymeric segments. Their participation in the grafting reaction is rather slow since the fabrics has to swell to assist the diffusion process of the monomer .entities to the active centers in the crystalline matrix to be grafted.
Moreover, during and after irradiation the liquid phase is rendered more viscous. Under such conditions the gel effect becomes more pronounced [15] and the formed radicals do not lose their reactivity but acquire longer life time and can remain trapped for extremely long periods, of the order of several days or even several months [16] . That behavior is encountered in systems which polymerize in a heterogeneous system containing acrylic acid, as in our work.
It is possible to conclude that grafting in the amorphous regions most probably leads to unstable copolymers in the medium and the reaction itself seems to be reversible, which is the case observed in copolymer A while grafting in crystalline regions probably leads to more stable graft copolymers due to their occurrence in an organized matrix of viscose rayon structure. However, the grafting reaction within the crystalline matrix occurs rather slowly to cope with the slow diffusion process of the monomer radicals through the ordered viscose rayon fabric matrix before being trapped.
On using DMF-water solvent together with viscose rayon and acrylic acid, the initial percent grafting gradually decreased until it became negligible 48 hours after the end of irradiation as could be observed in Figure 5. On increasing the storage time no further increase in the grafting yield was observed. This indicates that copolymer B was not formed when DMF-water solvent system was used. This could probably be explained by the fact that the solvent used does not sufficiently swell viscose rayon and that in turn reduces the accessibility of the embedded radicals in the crystalline regions of the solid ordered matrix to the monomer long living radicals in the viscous liquid phase.
In order to test the effect of polyvalent metal ions such as Co2+ on the grafting yield of acrylic acid onto viscose rayon after giving the required irradiation dose and storage, the previous experiment was repeated with the addition of 25 micromoles Co2+ to the irradiated solution. The results obtained are shown in Figure 6.
These results clearly show that the general behavior was quite similar to the behavior shown in Figure 5. However, the percent grafting yields recorded were higher than the corresponding yields when Fe2+ ions were present. This again demonstrated the accelerative effect of Co2+ on the grafting process. Moreover, copolymer A
![]()
Figure 6. Change of the percent grafting yield of acrylic acid onto viscose rayon with time of storage of samples after irradiation at room temperature, in presence of 25 micromoles Co2+ ions. Irradiation dose: 20 kGy: A + B: Decay curve of the graft formed during irradiation of viscose rayon fabric in presence of 10% acrylic acid and 90% of methanol/water solvent system (7:2) and 25 micromoles of Fe2+. The tubes were subjected to a gamma irradiation dose of 20 kGys after stopping irradiation. B―Formation curve of the acrylic acid graft formed after stopping irradiation. A―Decay Curve of the grafted polymer A initially formed during gamma irradiation with time of storage after irradiation.
is formed in presence of cobalt ions to a greater extent than in the presence of Fe2+ ions and at the same time the yield of copolymer B was less in presence of cobalt ions than in presence of Fe2+ ions.
Percent conversion of acrylic acid monomer in the irradiated viscose rayon samples during grafting
The per cent conversion of acrylic acid monomer in the irradiated samples was determined by acid base back titration of an aliquot of the liquid phase taken from the irradiated samples that were kept for increasing time intervals before analysis. The residual amounts of acrylic acid were almost the same in all samples and did not show any significant change with storage time after stopping irradiation. Thus 38 ± 1 mg of acrylic acid per ml were found in the liquid phases of the irradiated samples that originally contained 105 mg of acrylic acid per ml. The percent conversion amounted to 64%. On irradiating the liquid phase alone under the same experimental conditions, the percent conversion of acrylic acid amounted also to 63%. This clearly shows that while the percent monomer conversion is almost constant and depends only on the irradiation dose, the graft copolymer formation process is not only dependant on the irradiation dose but is also dependant on the time of contact of viscose rayon fibers with the liquid phase after being mutually irradiated and removed from the irradiation field This shows that the graft copolymer B continues formation after stopping irradiation due the continued interaction between the long living free radicals in the irradiated viscose rayon samples and the entrapped free radicals that remained reactive in the irradiated viscous liquid phase.
7) Effect of the thermal treatment of samples after irradiation
After irradiating the viscose rayon samples together with the liquid phase consisting of 10% Acrylic acid and 90% methanol-water solvent system (7:2) and using a dose 20 kGys, the sealed irradiation tubes were heated at different temperatures for 8 hours and then the graft yields were determined. From the results shown in Figure 7, it could be observed that the graft yield increased on heating the irradiated tubes at 50˚C, 70˚C and 90˚C by 16%, 25% and 58% respectively as compared to cold samples.
The same experiments were repeated but with the addition of 25 micromoles of Co2+ to the samples to be irradiated. The results are shown in Figure 8. It could be observed that on heating at 50˚C for four hours the percent grafting was found to increase by 30% as compared to the graft yield obtained without heating. On further heating for another four hours no significant changes in the graft yield was observed. On the other hand, on heating at 70˚C and 90˚C, extensive homopolymerization occurred.
![]()
Figure 7. Effect of post-irradiation thermal treatment of irradiated viscose rayon samples grafted with acrylic acid. The liquid phase: 10% Acrylic acid, 90% Methanol-water mixture (7:2) and 25 micro moles of Fe2+. Irradiation dose 20 kGy: Samples were heated at: 95˚C (a), 70˚C (b) and 50˚C (c).
![]()
Figure 8. Effect of post-irradiation thermal treatment (at 50˚C) on the graft yield of acrylic acid onto viscose rayon in the liquid phase consisting of 10% acrylic acid and 90% methanol: H2O solvent (7:2) containing 25 micromoles Co2+ ions. Irradiation dose: 20 kGy. a―Immediately after irradiation; b―48 hours after irradiation.
When the irradiated ampoules were kept for 48 hours after irradiation and then were heated for four hours at 50˚C the percent graft increased to be 40% greater than when no heating was applied, as could be also observed in Figure 8.
In partly crystalline and partly amorphous materials, such as cellulose and viscose rayon it is known that the free radicals formed within the crystalline regions are usually more firmly trapped [17] and consequently they readily react at elevated temperatures when most of the crystallites melt. This is not the case with radicals formed in the amorphous regions which can readily participate in many chemical processes such as grafting [18] .
The marked increase in the graft yield on heating the irradiated heterogeneous systems, depends on the possibility that the monomer molecules can more easily diffuse to the active free radical sites formed upon irradiation within the viscose rayon fibers. This process is facilitated by swelling of the polymer chains in the existing solvent.
Reactions involving free radicals are sensitive to temperature which affects the mobility of the radicals [19] . In addition, heating increases the swelling of the fibers and that increases the accessibility of the deeply seated free radical sites within the fibers to the diffusing monomer molecules. This probably explains the continuation of the grafting reaction after stopping gamma irradiation and heating afterwards.
It could be concluded that the increase in the grafting yield with time could be mainly attributed to the occurrence of the grafting reaction among the crystalline regions in viscose rayon due to the increased diffusion of monomer solvent combinations within the fibers to reach the deeply embedded radicals when the samples were thermally treated after irradiation.
8) Effect of heating and storage time on the grafting yields
It was interesting to investigate the combined effects of both heat and storage time on the grafting yields of acrylic acid on viscose rayon fabrics. Thus, certain weights of viscose rayon fabrics were placed in irradiation tubes together with 10 ml of a solvent system consisting of 10% acrylic acid and 90% of methanol/water solvent mixture (7:2) and 25 micromoles of Fe2+. The tubes were subjected to a gamma irradiation dose 20 kGys. After irradiation the sealed tubes were placed in a regulated water bath kept at 80˚C for 7 hours. After heating the samples were kept at room temperature for increasing time intervals. The graft yields were afterwards determined in each of the samples. The results are shown in Figure 9. It is clear that the graft yield increased gradually up to a maximum value at 37% after about 50 hours heating as compared to about 11% when no heating or storage of samples were applied. The marked increase in the grafting yield could again be attributed to the increased fiber swelling which increases the accessibility of the deeply seated free radicals within the fibers to the diffusing monomer molecules or radicals. Under the applied experimental conditions heating enhances the mobility of free radicals formed in the monomer molecules which in turn affects the overall grafting yield. The observed decrease in the grafting yield after 75 hours of storage of samples was accompanied with a marked degradation in the quality of the viscose rayon fabric and is accompanied with an increase in the viscosity of the liquid phase due to increased homopolymer formation.
5.2. Effects of the Grafting Yields on the Physical Properties of Viscose Rayon Fabrics Grafted with Acrylic Acid
The changes of the physical properties of the grafted viscose rayon fabrics have been studied. The results are
![]()
Figure 9. Change of the graft yield of acrylic acid onto viscose rayon samples, heated for 7 hours at 80˚C after irradiation (20 kGy) then kept for increasing time periods at room temperature. Graft yield (%) after irradiation: 10.6%.
given in the following:
1) Swelling (%) and moisture absorption %
Figure 10 shows the change of swelling (%) or moisture absorption (%) on increasing the graft yield (%). In methanol-water solvent swelling increased from 72% to 83% with a graft yield of 15% while moisture absorption slightly decreased from 7% to 5.9%. On using DMF-water solvent, swelling and moisture absorption sharply decreased with the graft yield more than 10%.
2) Tensile strength and elongation
Figure 11 shows that on increasing the graft % by about 10% both the tensile strength and elongation at break decreased to about half their values recorded for untreated fabrics. This reflects some deterioration of the properties of the fabric upon treatment.
3) Crease recovery angel
Figure 12 shows that the change of the crease recovery angle with the graft yield of the treated viscose rayon fabric. It is clear that the crease recovery angel was significantly improved on increasing the graft yield to 20%.
4) Dyeing properties of the grafted fabrics
![]()
Figure 10. Change of swelling (A) and moisture absorption (B) with the graft yield of acrylic acid onto viscose rayon using different solvent systems: a―Methanol-water (7:2); b―DMF-water (10:3). Irradiation dose: 20 kGy.
![]()
Figure 11. Change of the tensile strength and elongation at break (%) of viscose rayon sample grafted with acrylic acid with the graft yield. (A) Elongation at break%; (B) Tensile strength, kg/cm2. Solvent system: 10% acrylic acid and 90% methanol-water (7:2). Irradiation dose: 20 kGy.
The measured color strength of the dyed grafted fabrics on using three types of dyes are shown in Figure 13. It is clear that on using the direct dye or the disperse dye, no significant changes of the dye uptake were observed on increasing the graft yields. However, on using the basic dye the dye uptake was significantly increased on increasing the grafting yield up to about 20%.
6. Conclusions
1) Radiation induced grafting of acrylic acid onto viscose rayon has been successfully carried out.
2) Best grafting conditions were observed when viscose rayon was mutually irradiated with acrylic acid in methanol-water (7:2) solvent system with the monomer concentration around 10% of the liquid phase and irradiation dose around 20 kGy. Under these conditions the percent grafting amounted to about 13% and the percent monomer conversion amounted to about 65%.
3) Keeping the irradiated sample tubes after irradiation at room temperature resulted in a significant increase in the grafting yields. Thus, two copolymers were observed. Copolymer A was formed during irradiation and started to decay after stopping irradiation and copolymer B which mainly starts to be formed in the reaction tube after removing the samples away from the irradiation source.
4) On heating the reaction tubes after irradiation the percent grafting significantly increased. Heating at 95˚C for 4 hours resulted in a 50% increase in the graft yield as compared to the cold grafting yield while heating for 8 hours resulted in 67% increase than the value recorded when no heat was applied.
5) Best dying occurred on using the basic dye at a rather wide grafting yield range (up to about 20% grafting). On the other hand, grafting with acrylic acid did not improve the dyeing properties on using the disperse or the
![]()
Figure 12. Change of the crease recovery angle with the graft yield (%) of acrylic acid onto viscose rayon fabrics in the directions: a―Warp direction; b―Weft direction. Solvent system: 10% acrylic and 90% methanol-water (7:2). Irradiation dose: 20 kGy.
![]()
Figure 13. Change of the dye uptake with graft yield of acrylic acid onto viscose rayon using three different dyes: a―Disperse dye; b―Direct dye; c―Basic dye. Irradiation dose: 15 kGy. Solvent system used: DMF-H2O: (703).
direct dyes.
NOTES
![]()
*Corresponding author.