Onion Response to Added N in Histosols of Contrasting C and N Contents ()
Received 5 February 2016; accepted 13 March 2016; published 16 March 2016

1. Introduction
Onions (Allium cepa L.) are grown on nearly 9000 ha of Histosols in Quebec, Ontario and New York state. Depending on the C/N ratio, organic N amounts varied from 5000 to 27,000 kg N ha−1 in the top 20 cm of Histosols [1] . Reference [2] showed that combined effects of fertilization, drainage and mineralization produced 40 to 50 times more NO3-N in runoff water during the growing season under cultivated compared to uncultivated marsh in Ontario. Reference [3] reported N losses of 37 - 245 kg N ha−1∙yr−1 from Ontario Histosols with yearly concentrations varying between 15 and 43 mg NO3-N L−1 in surface waters. After mineralization of organic N into nitrate, the net nitrate accumulation reached 850 kg NO3-N ha−1 in New York Histosols [4] and 1400 kg NO3-N ha−1 in the Florida Everglades [5] . About 60% of the nitrate production accumulated in the 0 - 40 cm layer [1] . Growers practice is to add fertilizer N as preventive measure against under-fertilization. When soil N supply capacity is high over-fertilization must contribute to nutrient waste and water contamination [6] - [8] , especially for onion crops, due to low capacity of the root system to exploit soil N [9] .
In general, N requirements increase with yield potential [10] - [12] . Reference [13] found no significant effect of adding 22.4 kg N ha−1 to onion crops at yield potential of 41 Mg ha−1 in Quebec Histosols. Reference [1] reported no significant onion response at yield potential of 55 Mg ha−1. Reference [14] found that eliminating the N fertilization (56 - 112 kg N ha−1) could be very risky for early planted onions in New York Histosols at yield levels of 42 - 80 Mg N ha−1, because substantial quantities of mineral N were released later in the season. The discrepancy between the limited research results and growers’ practice is indicative of a large spectrum of soil properties and management options. Although a pre-side-dress-nitrogen test (PSNT) has been proposed to adjust N fertilization in Histosols [15] , there is still no soil test N (STN) to discriminate between responsive and non- responsive situations in Histosols with differential N mineralization potentials.
The N and C transformations in soils are closely related [16] . The C/N ratio thus allowed evaluating N mineralization or immobilization in Histosols [17] . Reference [18] suggested using a critical C/N ratio of 29 to separate the opposing processes of net mineralization and net immobilization in Histosols. In cultivated Quebec Histosols, C/N ratios were found to vary between 15 and 21, and were associated with the release of mineral N up to 620 kg N ha−1 in the upper 30 cm [19] . Reference [20] showed that N mineralization in Histosols depended not only on the C/N ratio but also on organic matter content. Reference [1] proposed using a compositional multi-ratio concept assuming that N mineralization was limited by C excess or C and N dilution in the residual soil mass computed as a filling value (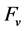 ) between 100% and analytical results (%C and %N).
) between 100% and analytical results (%C and %N).
Compositional data analysis provides tools to handle data closed to 100% that are distorted by redundancy of information, sub-compositional incoherence and non-normal distribution [21] . The isometric log ratio (ilr) is the most appropriate data transformation technique to avoid misinterpreting the results of statistical analyses of compositional data [22] [23] . Because ilrs are orthogonal to each other, a Mahalanobis distance (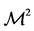 ) can be computed as STN index across C, N and
) can be computed as STN index across C, N and 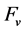 proportions to diagnose a given composition of Histosols against a reference one [24] . On the other hand, trials on N effect on crop yield can be synthesized using subgroup meta- analysis [25] - [27] . Allocating trials to STN subgroups and analyzing the effect size of N additions by meta- analysis could improve the accuracy of N fertilizer recommendations for onions grown in Histosols.
proportions to diagnose a given composition of Histosols against a reference one [24] . On the other hand, trials on N effect on crop yield can be synthesized using subgroup meta- analysis [25] - [27] . Allocating trials to STN subgroups and analyzing the effect size of N additions by meta- analysis could improve the accuracy of N fertilizer recommendations for onions grown in Histosols.
Our objective was to conduct a meta-analysis of multi-year and multi-site trials on yield response of dry onions to added N in Quebec Histosols using a compositional index as soil test N.
2. Material and Methods
2.1. Experimental Sites
The onion data set comprised 13 N fertilization trials conducted in Histosols of south-western Quebec, Quebec, Canada, between 2003 and 2006. Meteorological data were obtained from the Hemmingford station, Quebec (Latitude: 45˚4.200'N; Longitude: 73˚43.200'W; Altitude: 61 m). The length of the growing period averaged 120 d. The onion was irrigated during dry periods.
Plots were 3 to 8 rows in width and 6 to 8 m in length. Fertilizers were applied broadcast before sowing or in 2 split applications. There were four N rates up to 180 kg N ha−1 including a control treatment without N, allocated to three randomized blocks. Harvest date depended on the number of days required to meet commercial standards. Yields were measured in two central rows of 3 m in length. Plant density of cultivars Bastille, Fortress, Arsenal, Genesis, Frontier and Hamlet at harvest averaged 245,863, 325,650, 382,979, 449,173, 477,205 and 501,774 plants ha−1, respectively. Bulbs were classified as follows (Ø = diameter): extra-large (Ø > 76.3 mm), large (Ø 57.3 - 76.3 mm), medium (Ø 44.5 - 57.3 mm), small (Ø 31.8 - 44.5 mm), and discarded (too small, evidence of rot).
2.2. Soil Analysis
Soil samples were collected in the spring before fertilizer application and composited by block (three sub-sam- ples per sample). Soils were cleaned from roots and woody particles, air-dried to constant weight and sieved to < 2 mm before analysis. Soil pH was determined in a 0.01 M CaCl2 using a 1:4 soil to solution volumetric ratio [28] . Total C and N were determined by combustion using CNS-Leco 2000 [29] . Organic matter content was estimated assuming 58% C content. Elements were extracted using the Mehlich-3 method [30] and quantified by ICP-OES.
2.3. Compositional Data Analysis
The compositional space S of C and N analyses was described as follows [21] :
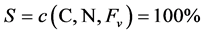 (1)
(1)
where 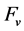 was computed by difference between 100% and analytical results (%C, %N) and c indicates closure of the simplex to the unit of measurement (here, 100%). Compositional data are relative to each other and thus inter-related. As inferred from Equation (1), any change in a given concentration (by adding more N for example) must affect the proportion of other components. Due to redundancy among components, there are D-1 degrees of freedom in a D-parts composition [31] . The ilr allows reducing D parts to D-1 orthogonally arranged variables. The D-1 ilr coordinates are computed as follows [22] :
was computed by difference between 100% and analytical results (%C, %N) and c indicates closure of the simplex to the unit of measurement (here, 100%). Compositional data are relative to each other and thus inter-related. As inferred from Equation (1), any change in a given concentration (by adding more N for example) must affect the proportion of other components. Due to redundancy among components, there are D-1 degrees of freedom in a D-parts composition [31] . The ilr allows reducing D parts to D-1 orthogonally arranged variables. The D-1 ilr coordinates are computed as follows [22] :
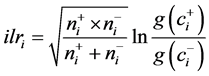 (2)
(2)
where i varies between 1 and D-1, ![]() and
and ![]() are the numbers of components in group
are the numbers of components in group ![]() at numerator and group
at numerator and group ![]() at denominator, respectively,
at denominator, respectively, ![]() is geometric mean across components in
is geometric mean across components in ![]() and
and ![]() is geometric mean across components in
is geometric mean across components in![]() . We selected the following two isometric log contrasts or balances between C, N and
. We selected the following two isometric log contrasts or balances between C, N and ![]() as follows: C (
as follows: C (![]() ) vs. N (
) vs. N (![]() ) representing the C/N ratio and the contrast between C and N (
) representing the C/N ratio and the contrast between C and N (![]() ) and
) and ![]() (
(![]() ) representing the dilution of C and N in the residual soil mass. For example, a soil containing 46.61% C and 2.09% N returns the following ilr values:
) representing the dilution of C and N in the residual soil mass. For example, a soil containing 46.61% C and 2.09% N returns the following ilr values:
![]() (3)
(3)
![]() (4)
(4)
Balance indices were computed as distances between a given composition and a reference one, as follows across results of Equations (3) and (4):
![]() (5)
(5)
![]() (6)
(6)
where ![]() and
and ![]() are the mean and standard deviation of a reference soil subpopulation defined using independent data from highly mineralizing Histosols (> 1 kg NO3-N ha−1∙d−1) in USA and Europe [32] . Reference ilr values (mean ± standard deviation) were computed as 1.899 ± 0.173 for
are the mean and standard deviation of a reference soil subpopulation defined using independent data from highly mineralizing Histosols (> 1 kg NO3-N ha−1∙d−1) in USA and Europe [32] . Reference ilr values (mean ± standard deviation) were computed as 1.899 ± 0.173 for ![]() and −1.391 ± 0.192 for
and −1.391 ± 0.192 for ![]() (Table 1). Because ilrs are orthogonal to each other, an STN index is computed as Mahalanobis distance (
(Table 1). Because ilrs are orthogonal to each other, an STN index is computed as Mahalanobis distance (![]() ) across results of Equations (5) and (6) as follows:
) across results of Equations (5) and (6) as follows:
![]()
Table 1. Compositional nutrient diagnosis norms for the three-component simplex (C, N, and Fv).
![]() (7)
(7)
To separate low-from high-N mineralizing Histosols, we selected the critical ![]() value of 5.5, computed as half the maximum
value of 5.5, computed as half the maximum ![]() value of 11 for net nitrification [32] . Net N immobilization was assumed to occur for
value of 11 for net nitrification [32] . Net N immobilization was assumed to occur for ![]() values >11, high-N net mineralization for
values >11, high-N net mineralization for ![]() values <5.5, and low-N mineralization for intermediate
values <5.5, and low-N mineralization for intermediate ![]() values (>5.5 and <11). Approximately halving or doubling a critical value is suggested to build soil fertility classes in view of interpreting soil test results to make fertilizer recommendations [33] .
values (>5.5 and <11). Approximately halving or doubling a critical value is suggested to build soil fertility classes in view of interpreting soil test results to make fertilizer recommendations [33] .
2.4. Statistical Analysis
Analysis of variance of marketable yields was conducted using the MIXED procedure using SAS version 9.2 for Windows [34] . Meta-analyses were conducted using Excel and formulas for random mixed models in [35] . The response ratio (RR) was computed as follows:
![]() (8)
(8)
The variance of RR was computed as follows [35] :
![]() (9)
(9)
where
![]() (10)
(10)
where
![]() (11)
(11)
where
![]() (12)
(12)
where ![]() is the pooled within group standard deviation,
is the pooled within group standard deviation, ![]() and
and ![]() are the numbers of observations in treatment and control, respectively (here,
are the numbers of observations in treatment and control, respectively (here,![]() ),
), ![]() and
and ![]() are treatment and control means, respectively, and
are treatment and control means, respectively, and ![]() and
and ![]() are standard deviations for treatment and control, respectively, computed as the square root of error mean square (
are standard deviations for treatment and control, respectively, computed as the square root of error mean square (![]() ) plus the term variance for the block effect (
) plus the term variance for the block effect (![]() ) which takes into account the standard error (
) which takes into account the standard error (![]() ) (Gaétan Daigle, professional statistician, University Laval, personal communication). Size effect in meta-analysis was declared significant at P < 0.10 for possible inclusion into the response model.
) (Gaétan Daigle, professional statistician, University Laval, personal communication). Size effect in meta-analysis was declared significant at P < 0.10 for possible inclusion into the response model.
3. Results and Discussion
3.1. Climate
Rainfall from May to August was higher in 2003 and 2006 compared to 2004 and 2005 (Figure 1). Climate was driest in 2005, especially in May and August. Irrigation was applied at need. However, the effect of climate on the response ratio could not be tested due to the limited number of N trials. Hence, STN was the only factor retained to build subgroups of onion response to added N.
3.2. Soil Properties
The soils covered a large spectrum of C and N concentration values and other properties (Table 2). Total soil C varied from 222.7 to 507.4 g C kg−1 while total N ranged between 11.6 and 25.3 g N kg−1 compared to 310 to 530 g C kg−1 and 19.0 to 40.0 g N kg−1 in [32] (Table 1). Organic matter varied between 12.9% and 29.4% and the soil C/N ratio between 18.9 and 29.3. Soil pH (CaCl2) varied from 4.5 to 6.7 therefore pHH2Ovaried from 4.7
![]()
Figure 1. Climatic conditions at the Hemmingford (Quebec) meteorological station near experimental sites (Columns represent rainfall and lines represent temperature). Source: Hemmingford station, Quebec (Latitude: 45˚4.200'N; Longitude: 73˚43.200'W; Altitude: 61 m).
![]()
Table 2. Soil properties and soil test N at the 13 onion experimental sites.
to 7.1 using the conversion equation of [28] , within ranges reported by [28] and [32] for Quebec Histosols. The ![]() values ranged between 1.1 and 19.6, hence reaching beyond the limit of 11 for net N immobilization suggested by [32] .
values ranged between 1.1 and 19.6, hence reaching beyond the limit of 11 for net N immobilization suggested by [32] .
3.3. Crop Response to Added N within Pre-Determined Soil N Fertility Classes
There were 9 sites in the ![]() STN group and 4 sites in the
STN group and 4 sites in the ![]() group (Table 3). Onion response to added N was smaller in the
group (Table 3). Onion response to added N was smaller in the ![]() compared to the
compared to the ![]() group. In the
group. In the![]() , onion response to N was significant at the 0.10 level adding 60 kg N ha−1. The onion crop in the
, onion response to N was significant at the 0.10 level adding 60 kg N ha−1. The onion crop in the ![]() group was responsive to added N at the 0.10 level of significance adding 180 kg N ha−1. The
group was responsive to added N at the 0.10 level of significance adding 180 kg N ha−1. The ![]() scanned a much larger range up to nearly 20 in the
scanned a much larger range up to nearly 20 in the ![]() STN group (Table 2) but the latter group could not be further partitioned due to the small number of observations.
STN group (Table 2) but the latter group could not be further partitioned due to the small number of observations.
On the other hand, in a Nova Scotia experiment on the Caribou bog where N was applied at rates of 0, 90, 180 and 270 kg N ha−1, reference [37] found that maximum onion yield of 50.5 Mg ha−1 was obtained with 180 kg N ha-1 for a ripening acid sphagnum peat soil cultivated five years after reclamation. In comparison, a maximum yield of 37.2 Mg ha−1 was obtained with 270 kg N ha−1 on a newly broken Histosol of similar origin. Although reference [37] did not provide soil C and N analyses, a former soil survey of the pristine Caribou bog [38] where their trial was conducted indicated that the upper soil layer made of the brownish fibrous mossy peat contained
0.91% of total N and 46% of total C (i.e., organic matter content = 79.3%), returning a ![]() value of 28.5, far beyond the upper limit of the model in Figure 2. Onion response was consistent with the peat ripening process where N concentration increases in the upper layer [39] resulting in lower
value of 28.5, far beyond the upper limit of the model in Figure 2. Onion response was consistent with the peat ripening process where N concentration increases in the upper layer [39] resulting in lower ![]() values, hence less N requirement.
values, hence less N requirement.
Reference [39] reported that Histosols showing C/N ratio of 29 could release 77 - 98 kg N ha−1 yr−1; more ripened soils showing C/N ratios of 23 - 24, 170 - 493 kg N ha−1 yr−1 while muck soils with C/N ratio of 18 could release 99 - 186 kg N ha−1 yr−1. The pattern of soil N mineralization capacity thus appeared to be quadratic. Indeed, total N and the C/N ratio could be effective STNs where ![]() content varies little such as in high-C peat materials [1] [17] [18] . Otherwise,
content varies little such as in high-C peat materials [1] [17] [18] . Otherwise, ![]() is a more suitable STN where Histosol compositions vary more widely within the limits of soil properties outlined in Table 1.
is a more suitable STN where Histosol compositions vary more widely within the limits of soil properties outlined in Table 1.
3.4. Provisory Onion N Recommendation Model
Significant (P < 0.10) trends of crop response to added N for treatments showing the highest RR (Table 3) in
![]()
Table 3. Response to added N (60 - 180 kg N ha−1) of onion grown in two fertility classes in Quebec organic soils. N.B. # is number of trials per group, STN is soil test N, N is total N, C/N is the C/N ratio, BRR is the back-transformation of ln (response ratio) into relative yield (treatment/control) and CI is confidence interval about RR.
ns, a: not significant and significantly different from BRR = 1 at the 0.10 level according to t test, respectively. Yieldt and Yield0: yields of treatment with added N and control (zero N), respectively.
![]()
Figure 2. Relationship between onion N requirements and STN fertility classes. The soil N fertility classes are indicated as Mahalanobis distance <5.5 and >5.5. The ranges represent the smallest and the highest Mahalanobis distance values in each STN class.
each STN class were selected to build a provisory N requirement model, i.e. 60 and 180 kg N ha−1 for the ![]() values of 3.16 (1.14 to 5.08) for the high-N mineralizing STN group and 10.55 (5.83 to 19.65) for the low-N mineralizing STN group, respectively.
values of 3.16 (1.14 to 5.08) for the high-N mineralizing STN group and 10.55 (5.83 to 19.65) for the low-N mineralizing STN group, respectively.
Bulb quality could also be considered in N management because onion flavor [40] [41] and susceptibility to diseases [9] and bulb rot [42] may be affected by N excess. Moreover, an oversupply of nitrogen during the growing period may promote excessive top growth resulting in bulb expansion and splitting, while late-season applications may promote top growth, delay maturity, and favor diseases [36] . Because rapid growth rate of seeded onion may not occur until 5 - 6 weeks after emergence, a PSNT test [15] may be further investigated as complementary diagnostic tool to allow seasonal adjustment of N fertilization to soil N supply capacity as defined by STN and to N leaching through rainfall and irrigation.
4. Conclusion
A compositional STN index that integrates C, N and ![]() into a Mahalanobis distance was calibrated against N requirements of onions grown on Histosols. The N requirements of onions appeared to be linearly related to the Mahalanobis distance
into a Mahalanobis distance was calibrated against N requirements of onions grown on Histosols. The N requirements of onions appeared to be linearly related to the Mahalanobis distance ![]() up to a value of 11. Onion crops grown in Histosols showing
up to a value of 11. Onion crops grown in Histosols showing ![]() values >5.5 required more N and yielded less in the control N treatment compared onions grown in Histosols with
values >5.5 required more N and yielded less in the control N treatment compared onions grown in Histosols with ![]() values <5.5. A provisory N requirement model was elaborated based on
values <5.5. A provisory N requirement model was elaborated based on ![]() values measured in the thirteen Quebec trials and on assumptions where no soil analysis was reported in the literature. Although strong response trends were found in this research work, onion N requirements could be further validated by including more sites showing low, intermediate and high
values measured in the thirteen Quebec trials and on assumptions where no soil analysis was reported in the literature. Although strong response trends were found in this research work, onion N requirements could be further validated by including more sites showing low, intermediate and high ![]() values under a larger spectrum of climate and soil conditions.
values under a larger spectrum of climate and soil conditions.
Acknowledgements
We thank the Conseil de Recherche en Pêche et Agroalimentaire du Québec, the Conseil pour le développement de l’agriculture du Québec (CDAQ), Phytodata Inc. (Sherrington, QC), the Fonds de recherche du Québec-Na- ture et Technologies (FRQNT), Biopterre Inc., the Natural Sciences and Engineering Research Council of Canada (NSERC-OG-2254), and the participating vegetable growers for financial support.
NOTES
![]()
*Corresponding author.