1. Introduction
Liquid Crystal Elastomers (LCEs) are new class of soft materials which combine the elastic properties of polymers and orientational properties of Liquid Crystals. They consist of the cross linked polymer chain networks and the crystalline ordering of Liquid Crystal. Thus it introduces a coupling between the orientation of the mesogens and macroscopic elastic deformations of the network [1] [2] .
LCE brings together three important properties: orientational order in amorphous soft materials, responsive molecular shape and quenched topological constraints, which helps to create many new physical phenomenon and have attracted significant interest from scientific community due to their fascinating thermal, mechanical, optical and electrical properties. The concept of LCE was put forward by the P.G Gennes, where as, the first LCE was synthesized by Finkelmann. LCEs are divided into nematic, smectic and others depending on the LC phases exhibited by the systems. In nematic phase, the polymer chains elongate, while in the isotropic phase, they recover a random coil conformation, which is driven by their entropy [3] -[5] .
In recent years the emphasis is being given on the synthesis and characterization of LCE. The studies of various physical stimuli, LCEs with thermo-responsive, photo-responsive and electro-responsive functions have been developed and LCEs as artificial muscles, micropumps and microvalves for microfluidic devices and opto- mechanical shutters have been actively explored [6] - [10] . Finkelmann et al. measured visco-elastic, opto-elastic and their behavior in electric field; Tajbakhsh et al. studied uniaxial monodomain Nematic elastomers. Terentjev et al. studied a wide range of photo sensitive materials; Dannio et al. studied thermo-tropic and elastic properties. In one way or other, many new physical properties of these soft materials have been discovered [11] -[16] .
In this work, we synthesized LCE using Finklemann procedure and the characterizations were performed by optical, thermal and mechanical techniques to understand the structure-property relation. These investigations confirm synthesis of LCE and the spontaneous effect on relative movement of their cross-linked end points results in the elastic response of the material which is very important for optimizing properties and achieving real applications.
2. Experimental
The synthesis of these elastomers were made Finklemann procedure by preparing partially crosslinked films in a centrifuge, highly swollen in toluene (2 - 3 ml per 1 g of material), reacting for 25 - 35 minutes before evaporat- ing the solvent. A careful study of reaction kinetics ensured that approximately 50% of crosslinks were established in the first stage of this preparation. The orientation is then fixed by the subsequent second-stage reaction, when the remaining crosslinks are fully established. The structure of polymer, monomer and cross linking agent is shown in Figure 1.
3. Results and Discussion
3.1. FTIR Spectroscopy Analysis
The synthesis of LCE were confirmed by FTIR spectra obtained from Perkin Elemer spectrometer in the spectral region ranging from 4000 to 400 cm−1. The FTIR spectra of synthesized LCE is shown in Figure 2. The bands in between 3445 and 2926 cm−1 is due to benzene C-H bond, bands at 2926 and 2857 cm−1 is due to long chain of -CH2- group, band at 1731 cm−1 is due to C=O group, band at 1606 cm−1 is due to C=C group, band at 1509 cm−1 is due to benzene (C=C), band at 1465 cm−1 is C-H deforming, 1256 cm−1 is due to Si-C bond, 1196 cm−1 is due to C-O (sample present in the group), 1069 cm−1 is due to C-O of benzene group, 1009 cm−1 is Si-O bonding, band at 763 cm−1 also confirms benzene in the sample. This spectrum confirms the synthesis of LCE.
3.2. SEM Analysis
The SEM images of the LCE at 10 μm and 30 μm scale for Everhart?Thornley (EHT) bias of 20 KV is shown in Figure 3. The most prominent feature is referred as the coarse structure of 10 μm scale. This is due to polymer network attached to the material. The structure of polymer, monomer and cross linking agent is also seen at 30 μm scale.
In these photographs, crosslinking are oriented axially and the anisotropic nodes lie in the different orienta- tions. SEM investigation revealed nematic nature of LCE. The surface of LCE also shows bend and give way under pressure or strain due to contraction. The elastic deformation related effects can be observed and also confirms the fact that degree of orientation of these materials can be controlled by varying crosslinking density.
3.3. FPSS Analysis
The LCE was heated by an indigenous electric heater characterized using He-Ne laser of power 2 mW. The light
scattered by the LCE sample was allowed to fall on the Fabry-perot Etalon and the angular diameters of the rings were measured at various temperatures. The experiment was repeated for five heating/cooling cycles for consistency. The graphical mapping of Angular diameter Vs Temperature for only one cycle is shown Figure 4. (other graphs can be provided if required). The abrupt variation in diameter shows mesophase transitin temera- ture of sample.
3.4. TGA Analysis
The thermal characterization of sample is performed by Perkin Elmer TGA. The analysis was made at heating rate of 10˚C/min. The weight is recorded as a function of increasing temperature from 30˚C to 150˚C. For TGA, the rate of mass change with time is described by the Equation:
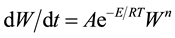
where: W is the dimensionless mass of sample subjected to degradation is time, A is a pre-exponential factor, E is the activation energy and n is the effective reaction order. The TGA graph of LCE is shown in Figure 5. It was observed that weight remains constant up to of 60˚C and again after 120˚C. The linear change in weight is due to spontaneous nature of the LCE.
3.5. DSC Analysis
DSC measures temperature differences in the heat flow path to the sample and reference material by constant rate heating. The DSC thermo graph of LCE for heating and cooling cycle is shown in Figure 6. The DSC signal can be expressed by the following Equation.
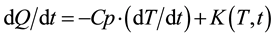
where, dQ/dt is DSC Total Heat Flow, dT/dt is Heating rate, Cp is Specific Heat Capacity of sample t is Time, T is Temperature and K(T, t) is Heat flow change with behavior.
The heat flow (in mW) was recorded up to temperature of 100˚C at a heating rate of 10˚C/min. The phase transition temperature observed by DSC is in agreement with FPSS and it also confirms spontaneous nature of the LCE characterized by TGA analysis.
3.6. Mechanical Studies
When mechanical forces are applied to LCE materials, they deform in reaction to those forces. The magnitude of the deformation depends on force. The length and breadth of sample were taken as 5 mm, thickness 1mm and area 25 mm2. The stress strain-graph of LCE studied by Force sensor is shown in Figure 7. The graph shows linear relation and hence obeys Hooke’s Law. The strain can be thought of as a normalized deformation. This shows elastic response of LCE.
4. Conclusion
The spectroscopic techniques confirm the synthesis whereas a thermal and mechanical characterization shows elastic and spontaneous nature of LCE. This is a unique property where a macroscopic shape change costs little elastic free energy. This spontaneous change of LCE leads to many applications such as thermo-mechanical actuators and artificial muscles. The control of optical birefringence of LCEs makes them suitable for opto- mechanical sensors and the fact that they are rather soft mechanically and still retain their shape as solids, makes them highly suitable for bifocal contact and intra-ocular lenses.
Acknowledgements
We would like to acknowledge the help and encouragement given to us by Dr. Anuradha Misra, Professor and Head, Department of Physics, University of Mumbai, Mumbai.