1. Introduction
Vigna unguiculata (L.) Walp (cowpea) is one of the most important food grain legumes of the family Fabaceae found in the tropics and sub-tropics between 35˚N and 30˚S across Asia, Africa, South America and Southern Europe [1] [2] . The center of diversity of wild cowpea (where you find the most variation) is in southern and southeastern Africa; the center of diversity of cultivated cowpea is in West Africa [3] . Today, the most primitive form of the plants is being distributed to other parts of the world and is now a broadly adapted and highly variable crop cultivated around the world. It is highly domesticated in West Africa [4] . In Nigeria, the vernacular names of some of the cowpea land race include “Olaudi”, “Akidi”, “Ileje” and “Ileje-Ajaka” [5] .
Cowpea is a drought-tolerant, warm-weathered crop that is well adapted in the dry savannah and it grows well in poor soils of sandy composition of 85% and less than 0.2% organic matter and low levels of phosphorus [6] . Cowpea is used as a multipurpose legume providing foliage leaf, green pods and grains. Cowpea is considered nutritious with a protein content of about 24.3%, carbohydrate content of about 63.64%, fat content of about 1.9%, fiber content of about 6.3% and water 8% - 9% [1] [7] . Cowpea seed coat contains flavonoid, an anti- oxidant found in fruits and vegetables which protect human cells from damage caused by radicals [8] . It improves soil fertility by fixing atmospheric nitrogen for soil enrichment [9] . The plant is shade-tolerant and therefore compatible as an intercrop with maize, millet, sorghum sugarcane and cotton. Cowpea is consumed as food especially its grain, green pod and fresh foliage green leaves. It is used as a cover crop as it has the ability to spread and provide ground cover, thus suppressing weeds and giving some protection against erosion. These attributes Coupled with its quick growth and rapid ground cover among other uses and importance have made the plant an essential component of sustainable subsistence agriculture especially in dry regions with little rainfall and poor acidic soil [10] . Its diversity of uses, nutritive contents and storage qualities has made the plant an integral part of the farming system in the West African region [9] .
Plant growth and development results from a combination of three processes at the cellular level: cell division (mitosis), cell expansion and cell differentiation [11] . Although cowpea is a multipurpose legume of high nutritional value, there is little or no information on the cytogenetics of its landraces. The continuous supply of new germplasm material as donor of various genes of agronomic importance is an important prerequisite for further improvement of cowpea cultivars especially as there are concerns that the yield peaks in major crops species including cowpea have been reached. Landraces of cowpea can undoubtedly contribute towards the development of germplasm pools. Cell division or mitosis and cell expansion determines cell number and cell size in a mature organ hence its yield [11] . Cytogenetic studies require knowledge of the most appropriate time for root tip collection for adequate observation of the chromosomes. This could best be determined through mitotic index studies. The mitotic index of a cell population has long been regarded as an important criterion for the growth and multiplication of the cells and tissues. One of the reasons for the mitotic indexing of species is to generate data which is important for breeding purposes [12] [13] . It becomes important therefore to study the mitotic index of the cow pea landrace, “Olaudi”.
2. Materials and Methods
Cowpea land race, “Olaudi” used in this study was obtained from Dr. Udensi Ugorji’s germplasm collection. Seeds were grown in petri dishes to obtain roots and the germination percentage was calculated and recorded. Root tips of “Olaudi” were collected at every hour, from 6 AM to 6 PM. Immediately after harvesting, the root tips were pre-treated with 8-hydroxyquinoline and kept at room temperature for three hours. The pre-treated root tips were rinsed in distilled water and fixed in a cold mixture of ethanol and acetic acid (3:1). Fixed samples were used after 12 - 24 h or transferred to 70% alcohol and stored in refrigerator until required. The fixed materials were hydrolysed in 1NHCl at 60˚C for 5 - 8 min. Meristematic portions werecut off unto clean slides in a drop of aceto-orcein stain. The meristem was gently tapped with a squashing rod. A cover slip was placed over the top and the excess stain was removed with filter paper by applying a firm thumb pressure. The slides were sealed with nail hardener [7] [14] . Prepared slides were viewed under the microscope at a magnification of ×100. Photographs of dividing cells were taken using a Canon Power Shot A630, 8.0 mega pixel digital camera with 4× optical zoom. Mitotic index for each root collection time was calculated from the number of dividing cells and the total number of cells using the formula:
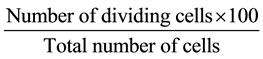 [7]
[7]
A complete Randomised Design was used in this study and data were collected based on the mitotic index at every hour from 6 AM to 6 PM. Data were subjected to Analysis of Variance (ANOVA) test. Significant means obtained were separated using Least Significant Difference (LSD) test. The germination percentage of was calculated using the formula
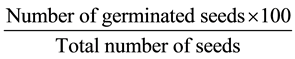
3. Results and Discussion
The results obtained showed that the germination percentage of cowpea (“Olaudi”) was 63.5% and mitotic divisions occurred at all intervals of the day, from 6 AM - 6 PM. From the hours of 9 AM and 1 PM, mitotic division increased rapidly and reduced significantly from 2 PM - 6 PM. Chromosome counts gave a diploid chromosome number of 2n = 22 (Figure 1)
The peak period of cell division was between 11 AM and 2 PM (Figure 2). The peak period for cells at prophase stage was observed at 12 PM, while that at metaphase stage was 11 AM. The peak period for cells at anaphase and telophase stage was observed at 2 PM and 11 AM respectively. Cell division occurred at all times during the day (Figure 2). The percentage of dividing cells was higher between 11 AM to 2 PM. At 6 AM, dividing cells started to increase with high percentage of prophase cells. The percentage of cells at prophase remained relatively high till about 2 PM when the percentage started decreasing. Metaphase cells increased after prophase and continued mostly between 7 AM to 12.00 noon. The number of cells at anaphase started to increase at about 11 AM and continued till about 2 PM.
Analysis of variance (Table 1) showed significant effect of time of harvest on mitotic index of “Olaudi” at P < 0.01. Root tips harvested at 1PM had the highest mitotic index of 65.3% while those harvested at 6 PM had the least mitotic index of 12%. Root tips harvested at 1 PM and 2 PM revealed cells with a significantly higher mitotic index than the cells of root tips harvested at 3 PM to 6 PM as shown in Table 2. Also, the mitotic index at 6 AM - 7 AM, was significantly different from the mitotic index at 9 AM - 11 AM, but there was no difference in the mitotic index of cells between 11 AM and 12 PM of root tip harvest.
![]()
Figure 2. Line graph and trendline showing proportion of cells at various stages of division during a 12-hour period of the day.
![]()
Table 1. Analysis of variance showing mean square of effects of harvest time on mitotic index of “Olaudi”.
*, **indicate significance at P < 0.05 and P < 0.01 respectively.
![]()
Table 2. Data for mitotic indices at various times of harvest.
Crop improvement is very important in the sustainability and security of food especially in Nigeria. This can be achieved by exploiting the potentials of landraces as sources of novel disease and abiotic stress resistant genes or combination of genes. Cell division is a continuous process and can be quantified by using the mitotic index. Mitotic index is mainly carried out to examine the proliferation status of cells and to determine if the plant is an actively dividing one. It is also used to quantify differences in cell division when an environmental parameter is changed [12] [13] . Cell division in “Olaudi” increased rapidly during the day which may be due to cellular metabolism and photosynthesis [15] . A diploid chromosome number 2n = 22 obtained in this study agrees with earlier results in cowpea as reported by [16] . The level of prophase stage was dominant at all the time intervals and it increased during the early hours of the day but reduced during the late hours. The processes of metaphase and anaphase, no doubt requires most energy, therefore, the observation of the highest number of metaphase and anaphase cells at 11 AM and 2 PM respectively corresponds to the period when most plants record a high photosynthetic level due to maximum availability of sunlight and thus, a high rate of synthesis of energy in the form of ATP [17] . Similar results have been reported in African yam bean [15] and in edible cocoyams [18] .
The proliferation status of a cell determines its germination rate hence the high germination percentage recorded in the plant. This is in line with the conclusions of [8] that cell division leads to increase in mitotic index as well as germination, growth and development of the plant which is based on several factors including oxygen concentration, light and moisture etc. In comparison, mitotic index in African Yam Bean peaked at 12 PM and 2 PM [15] , Treculia africana peaked at 4 PM [19] while cowpea peaked at 1 PM. This, according to [18] , implies that the peak of photosynthesis as well as mitosis may vary between species and can be exploited taxonomically. The information presented in this work is expected to serve as a first step for further cytogenetic research, exploitation and improvement of this crop.
Acknowledgements
Authors wish to acknowledge Dr. U. Udensi for providing some seeds of “Olaudi” landrace and the research support by Ms. Patience Ekong of the Department of Genetics and Biotechnology, University of Calabar.