1. Introduction
Intraoperative hemostasis during intracranial tumor surgery is one of the most important aspects of the surgical procedure. Hemostasis is necessary to keep a clean operative field, to prevent blood loss, and to prevent a postoperative hemorrhage. The surgical resection cavity should be completely dry of blood before dural closure and even slight oozing should not be accepted.
One of the most widely practiced methods of final hemostasis of the intracranial surgical resection cavity is to cover it with a sheet of oxidized cellulose [1] [2] . The actual hemostasis has then already been performed generally using bipolar forceps and the resection cavity is just covered to stop or prevent small capillary bleedings. One or two layers of oxidized cellulose are usually left behind in the resection bed.
The incidence of complications of this technique is relatively low considering the frequent usage of oxidized cellulose. However, cellulose may swell in the postoperative period and cause severe neurological deficits [3] - [6] . Granuloma formation has also been reported as a complication of the use of cellulose [7] . Therefore, large quantities of cellulose should not be left in bony canals or near the optic nerve.
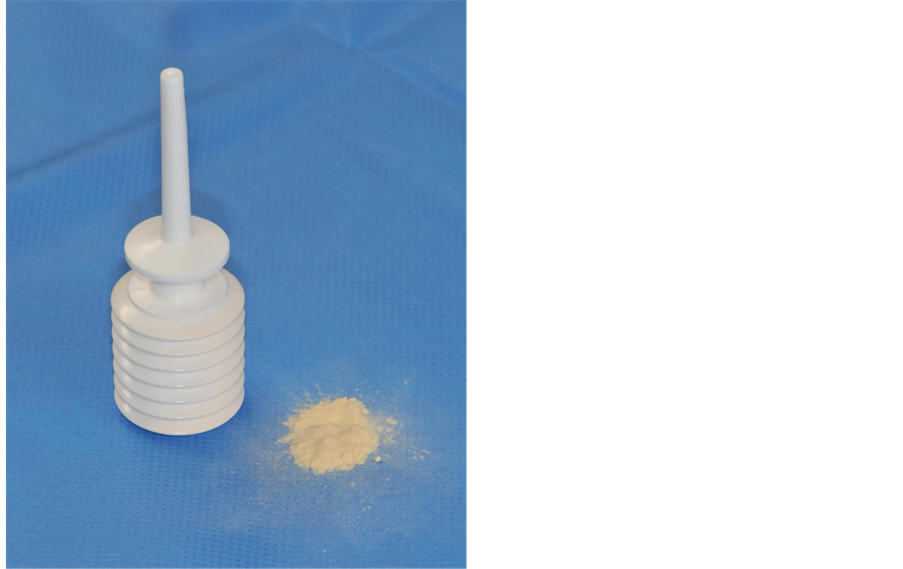
GelitaCel Ca Powder™ (Gelita Medical GmbH, Eberbach, Germany) is a powder form of oxidized cellulose enriched with calcium applied with a nozzle (figure 1). Once applied, it has less volume than the regular cellulose. The surplus is readily washed out without disrupting the hemostatic fibrin clots in the resection cavity. For these reasons there are potential advantages in its use.
In this clinical report, we describe the use of this form of cellulose powder for hemostasis purposes in intracranial neurosurgery.
2. Methods
All consecutive craniotomy patients operated by single surgeon between January 2010 and June 2012 were included in the study. The presence of a resection bed was required, so patients without a resection bed such as aneurysm patients were excluded. Also patients presenting with a spontaneous or traumatic intracerebral hematoma or contusion were excluded as the goal of this type of surgery is not always a blood free resection bed.
All patients included in this clinical report (n = 107) underwent either an elective or an emergency craniotomy and were operated by one single surgeon (TM). The patient characteristics are summarized in Table1 In all elective patients, antiplatelet and anticoagulation medication was stopped at least 7 days prior to surgery (and corrected if necessary) and the laboratory hemostasis parameters were checked before surgery.
Surgical Technique
All craniotomies were performed using a high-speed drill. Usually, one or two burr holes were made with a 3 mm high-speed drill and the craniotomy was completed with a craniotome. In each patient, the lesion (such as tumor or arteriovenous malformation, AVM) was removed using standard microneurosurgical techniques under magnification. After completing the resection of the lesion, hemostasis of the resection cavity was accomplished mainly using bipolar forceps coagulation with the patient being normotensive (i.e. blood pressures around 120/80 mmHg). The resection bed was carefully rinsed with physiological saline. After complete or near complete hemostasis (figure 2(a)), the resection bed was covered with cellulose powder by dispersing it in a thin layer over the resection bed using a single nozzle dispenser (figure 2(b)). The cellulose powder was left in situ for approx. four minutes and the resection cavity was again inspected to confirm complete hemostasis. If no bleeding was detected, the cavity was then generously rinsed with warm saline in order to remove superfluous powder (figure 2(c)). If a bleeding was detected, hemostasis was performed again with bipolar forceps and additional application of the powder oxidized cellulose.
The dura was closed (either primary or by a dural substitute) by 4 - 0 prolene sutures and the suture line reinforced with fibrin sealant (Tisseel, Baxter AG, Vienna, Austria). In large craniotomies, one central tackle suture was used to attach the dura to the bone flap. The bone flap was fixed using cranial fixation devices as Cranial Loop (Neos, Barcelona, Spain) or Bioplates (Synthes, Switserland).
All patients had a postoperative CT-scanning or MR imaging scanning within 24 hours after surgery according to routine clinical practice. To grade the presence of blood on the postoperative imaging, the following classification was made (see also Table 2):
1) no blood in the resection cavity;
2) minimal trace of blood in the resection cavity;
 (a)
(a) (b)
(b)
Figure 1. Application nozzle (a); and Detail of the cellulose powder (b).
 (a)
(a) (b)
(b) (c)
(c)
Figure 2. Wound resection bed before application (a); Application of the powder (b); and Resection bed after rinsing with saline (c).

Table 1. Summary of the operated patients (n = 107).

Table 2. Classification of postoperative hematoma on CT scanning and/or MR imaging.
3) blood filling the resection cavity without mass effect;
4) blood filling the resection cavity with mass effect.
The maximal diameter of blood (i.e. hematoma) in the resection cavity was measured in all patients. All surviving patients were seen on the outpatient clinic six weeks, three, and six months after surgery.
3. Results
Of the 107 operated patients, 96 (90%) had no or minimal blood in the resection bed on postoperative CT scanning or MR imaging (Figure 1). Of those 96 patients, 69 (64% of the total) patients had no blood at all while twenty-seven (25%) patients had a small amount (trace) blood in the resection cavity (figure 3).
In the remaining 11 patients, 8 patients (7.5%) had blood filling the resection cavity without mass effect. In 3 patients (2.8%), the blood accumulation resulted in mass effect and these patients were reoperated and the hematomas surgically removed. Two of those had a hemorrhage outside the resection cavity but subcutaneous and epidural blood breaking into the cavity, and one patient had a true postoperative hemorrhage in the resection cavity. In this patient, aspirin had been erroneously restarted one day after surgery.
One patient experienced an epileptic seizure directly after surgery. This was a patient with a previously embolized AVM in the medio-temporal lobe on the left side. This patient had no blood in the resection cavity as a possible explanation of the epileptic seizure.
None of the patients had complications related to the use of GelitaCel Ca Powder™ (no granuloma formation, no intracranial infection, no hydrocephalus).
The number of patients and maximal cross section of blood in the resection cavity of the 107 patients is presented in Table3
4. Discussion
Oxidized cellulose was first described by Yackel and Kenyon of the Eastman Kodak Laboratories in 1942 [8] . The hemostatic use of oxidized cellulose was first introduced by Frantz in 1943 [9] [10] , while oxidized cellulose was launched in 1960. The use of oxidized celluloses has gained wide spread acceptance since the 50’s and 60’s in neurosurgery, mainly to stop oozing in the cerebral resection cavity. It is a common practice for neurosurgeons to cover the brain bed cavity with sheets of cellulose, even if it does not actively bleed [1] . This preventive hemostasis technique has never been proven scientifically and it seems it is more a habit and a psychological reassurance for the neurosurgeon. Nowadays oxidized cellulose (branded as Surgicel or Oxycel) or oxidized cellulose (branded as Gelitacel) is a widely accepted and used hemostat both for cranial and spinal surgery [1] . Despite its wide acceptance, not a single randomized study has been published proving its efficiency in preventing a postoperative hemorrhage. Some neurosurgeons do not leave anything in the resection cavity to prevent a postoperative bleeding, while others cover the resection bed with cellulose or other forms of hemostats such as gelatin. Which of these techniques is used depends on local habits and beliefs. On the other hand it is clear that application of cellulose may stop a small oozing from the brain surface. More heavy bleedings are however not stopped by application of cellulose.
The mode of action of oxidized (regenerated) cellulose is not fully elucidated but it is believed to be both physical (mechanical compression and blood absorption) and chemical (surface interactions with proteins and platelets, and activation of the intrinsic and extrinsic pathways) [10] -[12] . Oxidized cellulose has a low pH that is also thought to be responsible for its hemostatic and bacteriostatic effect [11] [12] . Cellulose has to be applied manually and slightly pressed against the brain surface.
Absorption of oxidized cellulose is thought to require at least 3 to 4 weeks (for oxidized cellulose around 2 weeks), but greatly depends on the volume left in situ [13] . It is a common finding during re-operation
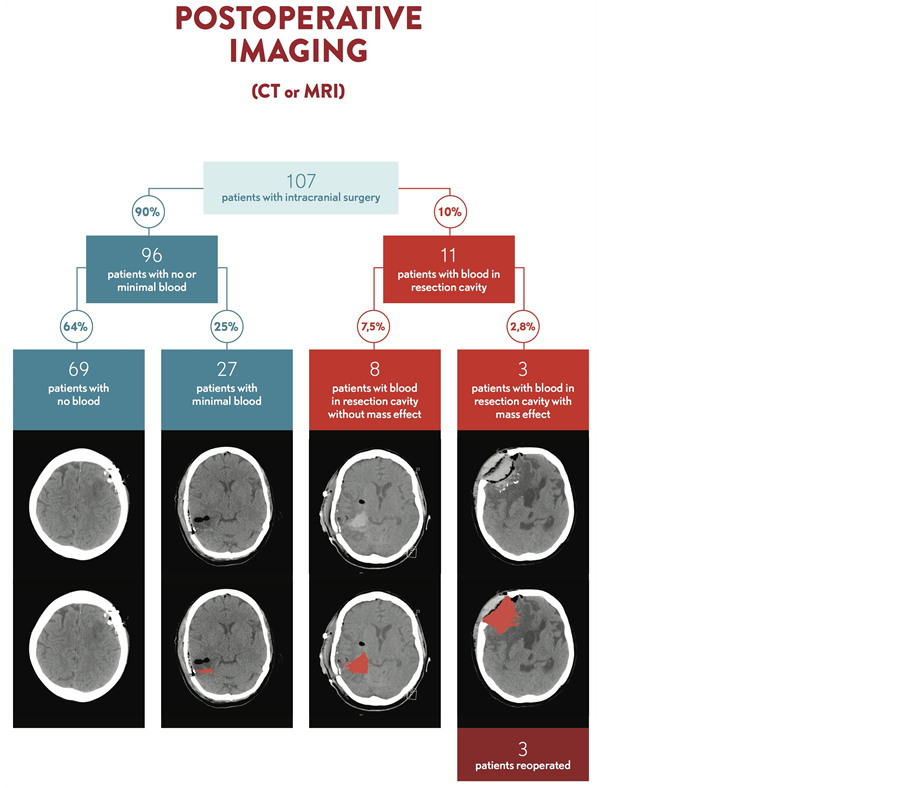
Figure 3. Summary of the postoperative imaging results of the 107 operated patients.

Table 3. Number of patients and maximal cross section of blood in the resection cavity (in mm) for different groups of patients.
that significant volumes of celluloses remain present for a long time, even more than 5 weeks (personal observation). As with any other foreign body material, the less material is left behind, the faster absorption and less inflammation and granuloma is formed. The incidence of complications is relatively low considering the frequent usage of the product. However, swelling leading to neurological deficit has been reported both in the neurosurgical, thoracic, and orthopedic literature [3] -[6] .
From a chemical point of view, both oxidized cellulose and oxidized cellulose contain the same active agent: 6-carboxycellulose. Regeneration refers only to the raw material used for oxidized cellulose. Rayon/viscose used for manufacturing of oxidized cellulose is an “artificial” material—it is known also as an “artificial silk”—originating from wood pulp of very different cellulose sources and converted into rayon/viscose [13] . Oxidized cellulose such as used in this study originates from natural plant based cotton is being purely natural cotton material [14] . The structure of natural cotton is thus basically different from rayon and viscose fibers. For hemostatic purposes, the natural cotton has an increased surface due to the irregular natural structure of the cotton fibers in comparison with rayon/viscose [14] .
The manufacturers of cellulose for hemostasis purpose state in their product information leaflet that “it must always be removed from the site of application when used in, around, or in the proximity to foramina in bone, areas of bony confine, the spinal cord, and/or the optic nerve and chiasm regardless of the type of surgical procedure because cellulose, by swelling, may exert pressure resulting in paralysis and/or nerve damage”. However, the common practice among neurosurgeons is to leave the cellulose in the majority of cases in situ, as removing the cellulose can reinitiate the bleeding.
The powder form of cellulose as described in this paper originates from the oxidized form of cellulose (pure natural cotton) and is enriched with calcium [15] . Various co-factors are required for the proper functioning of the coagulation cascade, most important of them being calcium ions and vitamin K. They affect almost every aspect of the clotting process. All three pathways (intrinsic, extrinsic, and common) require calcium ions and vitamin K and any disorder that lowers their plasma concentrations will impair blood coagulation.
The disadvantages of regular cellulose are partially compensated by the powder form: the application is atraumatic (no-touch technique) as the powder is sprayed on the surface and it follows exactly the surface of the resection bed. If necessary, the powder can be a little bit pressed in the tissue with a patty such as the regular form of cellulose. Once applied, the powder creates a sticky film on the surface that bleeds. This is also an additional mechanism of hemostasis as a more concentrated plug and coagulum is formed. On saturation with blood the powder crystallizes into a sticky, yellowish/grey layer. The product does not stick to surgical gloves or instruments.
The amount of powder required for hemostasis is less that a regular sheet of cellulose, and the removal of the powder is again atraumatic. By rinsing with saline, the powder particles that are not incorporated in a hemostatic clot are readily washed out and the ones that are “trapped” in the hemostatic meshwork stay in situ despite rinsing. This results in the situation that only the required (but minimal) amount of powder is left behind. Because the powder forms just a very fine thin layer on the brain, it is unlikely that the powder will close natural openings in ventricular spaces such as foramen of Monro, aquaduct, and obex. Sofar we have not experienced this complication. If the powder is applied too thick or superfluously, the superficial layers can be gently sucked away with the suction tube without disrupting the deepest layer. The powder form of oxidized cellulose is especially suitable for hemostasis at delicate and irregular anatomical structures such as cranial nerves, brain stem, and spinal cord in which surgical manipulation or bipolar forceps coagulation may lead to neural or vascular damage. The powder form has the advantage to provide a superior contact with the bleeding source in the presence of complex three-dimensional anatomic structures, which enhances its hemostatic properties. The powder is not suitable for arterial bleedings or heavy venous bleedings such as in the cavernous sinus. In these situations, the thin layer of powder is too fragile to efficiently provide hemostasis.
The postoperative bleeding percentage of 2.8% percent in this serie compares well with the reported percentages in the literature varying between 0.8% - 7.1% [16] -[22] . In our series of 107 patients, all patients had a mass lesion resected, creating a resection surface and cavity. This is essentially different from patients having intracranial surgery without a resection bed such as clipping of an aneurysm or microvascular decompression. It is well known that meningioma surgery carries a higher risk for a postoperative hematoma, mainly in the elderly patient [17] . This is well reflected in our series of patients. All three patients in our study with a postoperative hematoma were operated for a meningioma, and one of them has received antiplatelets medication (Aspirin) one day after surgery by mistake. Other important factors for developing a postoperative hematoma are abnormal hemostasis and coagulation parameters, intraoperative or immediate postoperative hypertension, incomplete tumor removal, age > 70, hypoxia and hypercapnia, coughing, and difficult intraoperative hemostasis with blood loss of more than 500 cc [16] -[19] [22] [23] .
The size of the resection cavity probably determines the amount of blood that can accumulate inside it. We found that in 7.5% of the patients, the resection cavity was filled to some degree with blood and blood like fluid. On CT scanning, it is difficult to discern whether it is purely blood or blood mixed with CSF and rinsing fluid. In most of these cases, it is likely a mixture as the signal was not always highly hyperintense (such as with classical postoperative hemorrhage). 25% patients had a small amount (trace) blood in the resection cavity. In this group, the largest diameter of this “blood” ranged from 2 mm to 13 mm.
The limitation of the present study is that 1) it is not a comparative study comparing the powder form to regular oxidized cellulose but 2) more a technical report on the novelty and the use of this product. As a consequence, this study does not intend to demonstrate any superiority of the cellulose powder over other hemostatic materials and products but reflects only our personal experience with this product. Moreover, the study reports a limited number of patients (n = 107) in s single center in a limited number of intracranial pathologies.
5. Conclusion
Although this is a preliminary report without a control group, we suggest that GelitaCel Ca Powder™ is an easy and safe product for hemostasis in intracranial surgery. It has some distinguished advantages over regular oxidized cellulose such as the no-touch technique of application and leaves no excess hemostatic material behind.
Acknowledgements
The authors thank Chris Mott, Fons Van Dijck, and Andy for the intraoperative photographs.
Financial Interest
The authors have no personal or institutional financial interest in drugs, materials, or devices described in this submission.
NOTES
*Corresponding author.