Ruminal degradability of Guinea grass silage inoculated with Streptoccocus bovis isolated from bovine rumen combined or not with com wheat bran ()
1. INTRODUCTION
In central Brazil due to good weather conditions recorded during the summer, there is a large production of perennial forage plants. However, due to seasonal climate variations, it is necessary to store the perennial forage surplus obtained when the weather is favorable, to meet the animal requirements throughout the year. The potential of a plant for silage depends on the original moisture level, which should be close to 70% of the soluble carbohydrate content (more than 8% for dry matter) and on a low buffering capacity, which should not resist the reduction of pH to between 3.8 and 4.2 (McDonald et al. [1]). However, when ensiled in early stage of vegetative development they have a high nutritional value, but have low dry matter content, increasing buffering capacity and low levels of soluble carbohydrates, which make it difficult to obtain good quality silage (Coan et al. [2]; Zanine et al. [3]).
Given this reality, Coelho [4] notes that with advances in the agro-industrial sector, by-products arose from the processing of cereals, fruits and food products, as well as bacterial inoculants that have been suggested as an alternative to improve the fermentation pattern, nutritional value, degradability of silages and, therefore, improvements in animal performance.
According to Ferreira [5], the first condition for the use of an inoculant to result in improvements in the control of undesirable microorganisms and in the fermentation pattern of silages is related to the amount of epiphytic lactic acid bacteria in the plants to be ensiled. The epiphytic lactic acid bacteria are the most abundant and active in the fermentation process (Penteado et al. [6]). Chunjian et al. [7], argues that the presence and quantity of these groups of bacteria determine if the fermentation is appropriate and if there is a need to apply microbial inoculants.
Among the lactic acid bacteria Streptococcus bovis stands out, it is a lactic acid bacteria isolated from the rumen, with features that allow it to be used as a silage inoculant. Its main feature concerns the specific growth rate of this species, 30% higher than the growth rate of the species of lactic acid bacteria used as inoculants for silage, which suggests that it may act as the starter culture of the fermentation process (starter), promoting rapid decrease in pH of the silage (Jones et al. [8]; Oliveira et al. [9]; Zanine et al. [10]; Ferreira [5]).
Although the effectiveness of the Streptococcus bovis is reported in the scientific literature (Jones et al. [8]; Oliveira et al. [9]; Zanine et al. [10]; Ferreira et al. [11]; Ferreira et al. [12]), the alleged use of a source supplier of high quality carbohydrates, such as wheat bran, can further optimize the initial growth of this microbial population, allowing a more rapid and efficient fermentation.
Given the above, the objective was to evaluate the ruminal degradation in situ of the dry matter, crude protein, neutral detergent fiber and acid detergent fiber of Guinea grass silage, inoculated with Streptoccocus bovis isolated from bovine rumen and combined or not with wheat bran.
2. MATERIALS AND METHODS
2.1. Preparation for Ensilage and Treatments
The experiment was conducted at the Department of Animal Science, Federal University of Viçosa, located in Viçosa—MG, Brazil, between December 2008 and April 2009. The city of Viçosa is located at 20˚ and 45' south latitude, 42˚ and 51' west longitude and 657 m altitude, with average rainfall of 1341 mm, of which about 86% take place from October to April.
The study used a Panicum maximum cv. Guinea grass on approximately 0.5 ha paddock. The grass was cut uniformly, fertilized with 50 kg/ha of nitrogen and potassium in the form of ammonium sulfate and potassium chloride, respectively. Harvesting and ensiling of grass was done 65 days after the uniform cut.
The experimental design was completely randomized with six treatments and six repetitions: Guinea grass silage, Guinea grass silage inoculated with 10% of wheat bran, Guinea grass silage inoculated with 106 cfu/g of strains of Streptococcus bovis JB1; Guinea grass silage inoculated with 106 cfu/g of strains of Streptococcus bovis JB1 plus 10% of wheat bran; Guinea grass silage inoculated with 106 cfu/g of strains of Streptococcus bovis HC5; Guinea grass silage inoculated with 106 cfu/g of strains of Streptococcus bovis HC5 plus 10% of wheat bran.
Grass was chopped with a stationary chopper, adjusted to create particles of lengths between 2 - 3 cm. The inoculant was applied to the grass, sprayed in layers of approximately 20 cm, as well as wheat bran. After homogenization, the material was ensiled into silos with a capacity of 15 liters.
2.2. Microbial Inoculant Preparation and Fermentation Profile
During the preparation of the inoculants, the cultures were grown in MRS medium (De Man, prayers and Sharpe) and subject to three successive activations, on the day before ensiling. Subsequently they were cultivated in MRS solid medium for the counting of the microbial populations (Santos [13]). Based on the result of the bacterial concentration in each inoculant, it was determined the necessary dilution for each inoculant, 106 cfu/g of fresh forage, based on preliminary counting in MRS Agar medium. Before ensiling, the cultures were again activated and diluted in distilled water at the time of ensiling, in order to achieve the predetermined concentrations.
At the opening of the silos, 45 days after ensiling, subsamples of approximately 25 g were collected for pH analysis, to which were added 100 mL of water and, after resting for two hours, the pH reading was made, using a potentiometer. In another subsample of 25, it was added a 200 mL solution of H2SO4, 0.2 N, remaining at rest for 48 hours to then perform filtering using a Whatman 54 filter. This filtrate was stored in the refrigerator for subsequent analysis of ammonia-N (Bolsen et al. [14]). To determine the organic acids, approximately 25 g of fresh silage was diluted in 250 mL of distilled water and homogenized in an industrial blender for 1 minute. The resulting aqueous extract was filtered through filter paper and 100 mL were acidified with H2SO4 50% and subsequently filtered through fast filter paper (Kung Jr. & Ranjit Jr. [15]). In 2 mL of this filtrate it was added 1 mL of 20% metaphosphoric acid solution and 0.2 mL of 1% carbolic acid solution, used as internal standard.
The determination of lactic acid, acetic, propionic and butyric acid were performed by high performance liquid chromatography (HPLC), brand Schimadzu SPD-10, with a wavelength of 210 nm. It was used a C-18 column, reversed-phase pressure of 168 kgf and flow of 1.5 mL/ minute. In Table 1 it can be verified the average values of the fermentation pattern of the Guinea grass silages with added Streptoccocus bovis combined or not with wheat bran.
2.3. Chemical Composition
The silage samples were dried in a forced air circulation oven, set at 65˚C for 72 hours. They were subsequently ground Willey-type and it was determined the dry matter (DM), mineral material (MM), crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber
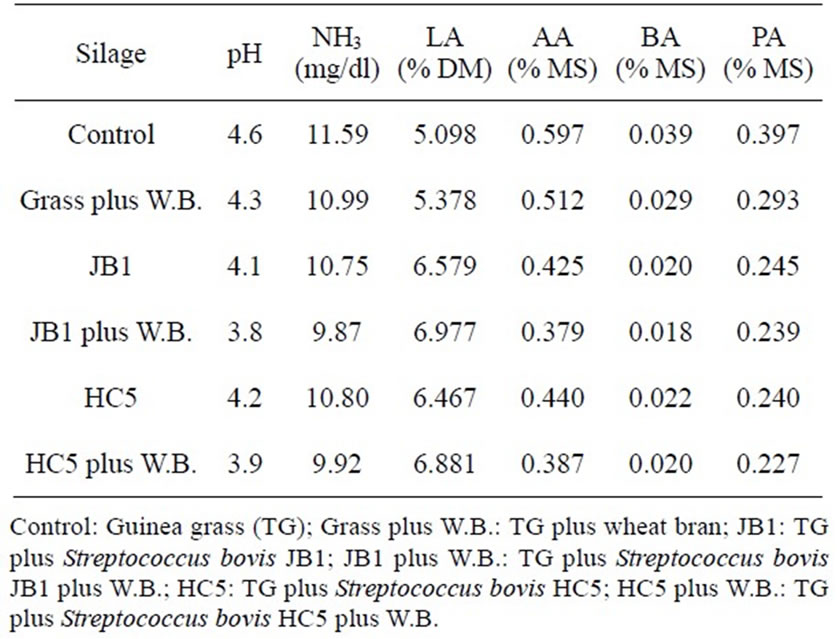
Table 1. Average pH values, NH3, lactic acid (LA), acetic acid (AA), butyric acid (BA) and propionic acid (PA) of Guinea grass silage.
(ADF) and hemicellulose (HEM) (Table 2), using methods described by Silva and Queiroz [16].
2.4. Ruminal Degradability in Situ Nutrient
For ruminal degradability the grinded samples were placed in nylon bags in order to provide 10 to 20 mg sample/cm2 us able area (Nocek [17]) for ruminal incubation in situ. The feeds, in triplicate, were incubated in the rumen of three fistulated bred, with an average weight of 300 kg, kept on pastures of Brachiaria decumbens grass. The bags were inserted into the rumen at the same time, and removed 0, 2, 4, 8, 16, 24, 48, 72, 96 and 144 hours after incubation of the bulky, according to NRC [18].
It was determined the Silages degradation of dry matter (DM), crude protein (CP), neutral detergent fiber (NDF) and acid detergent fiber (ADF) (Nocek [17]). In the estimation of kinetic parameters of DM and CP it was used the first-order asymptotic model Deg(t) = a + b (1 − e−ct), proposed by Orskov and McDonald [19], where Deg (t) represents the degradability of the food constituent’s (DM, CP) disappearance, expressed in percentage, “a” is the fraction of the food soluble in water at time zero; “b” is the fraction of water-insoluble but potentially degradable in the rumen at a given time, “c” is the degradation rate of the fraction potentially degradable in the rumen (b); “t” is the incubation time (hours).
In the estimation of degradability of NDF and ADF it was use d the model proposed by Waldo et al. [20], R(t) = D (e−ct) + I, where R (t) represents the incubation residue at the time t (hours), D is the NDF fraction potentially degradable in the rumen, c is the fraction D degradation rate; “I” is the fraction of NDF that is non-degradable.
The degradation curves of DM, CP, NDF and ADF of
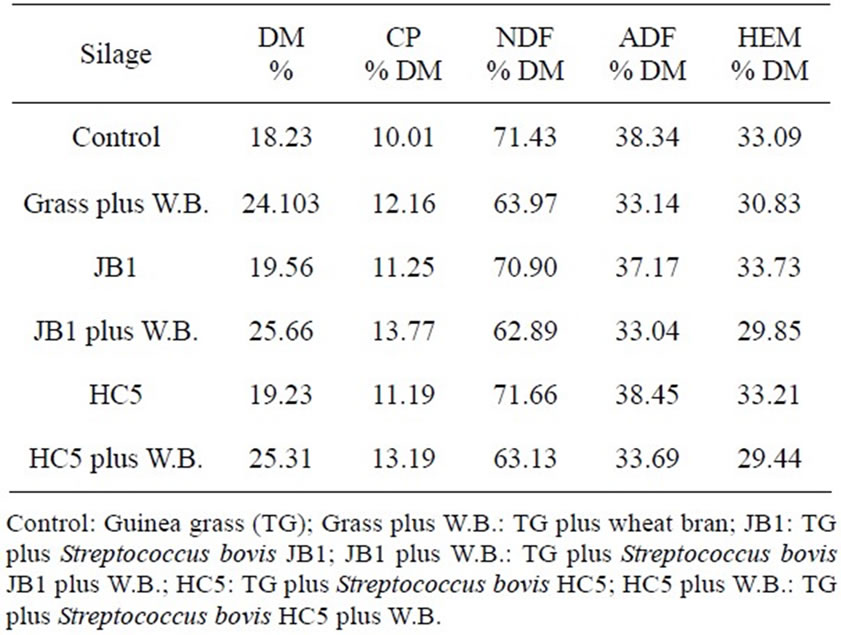
Table 2. Chemical composition of Guinea grass.
the foods studies, for each animal used, were subject to adjustment by the respective models, using the procedure of “regression Marquardt” from the software SAS [21], which makes possible to obtain the estimates of the parameters studied.
3. RESULTS AND DISCUSSION
In Table 3 are the values of the parameter estimates of ruminal degradability of dry matter and crude protein from the Guinea grass silages with additives of Streptoccocus bovis combined or not with wheat bran. Whereas the fraction “a” represents the portion of the plant that is readily available to rumen microorganisms it is possible that the microbial inoculant may have contributed to the increase of this fraction in silages, especially in silages inoculated with Streptococcus bovis JB1 and HC5 with added wheat bran, as these inoculants resulted in higher values of dry matter 32.76%, and 32.17%, and crude protein 38.28% and 37.89%, respectively (Table 3) due to good quality of silage studied (Tables 1 and 2).
Such result was probably due to the inclusion of wheat bran in silages inoculated with Streptococcus bovis, thus promoting reduction in the grass moisture level, helping to reduce the pH and ammonia-N (Table 1), favoring the growth of lactic acid bacteria (Table 1) over the enterobacteria, increasing the formation of lactic acid (Table 1) reducing losses of dry matter (Table 2). According to Knicky [22], wheat bran increases the contents of soluble carbohydrates and dry matter, and promotes the reduction of anionic substances such as salts of organic acids, nitrate, sulfate, among others, favoring the preservation of the ensiled material and consequently improving the degradability of dry matter and crude protein (Table 3).
These results corroborate with those obtained by Ferreira et al. [12], who verified the superiority of fraction “a” of dry matter and crude protein in elephant grass
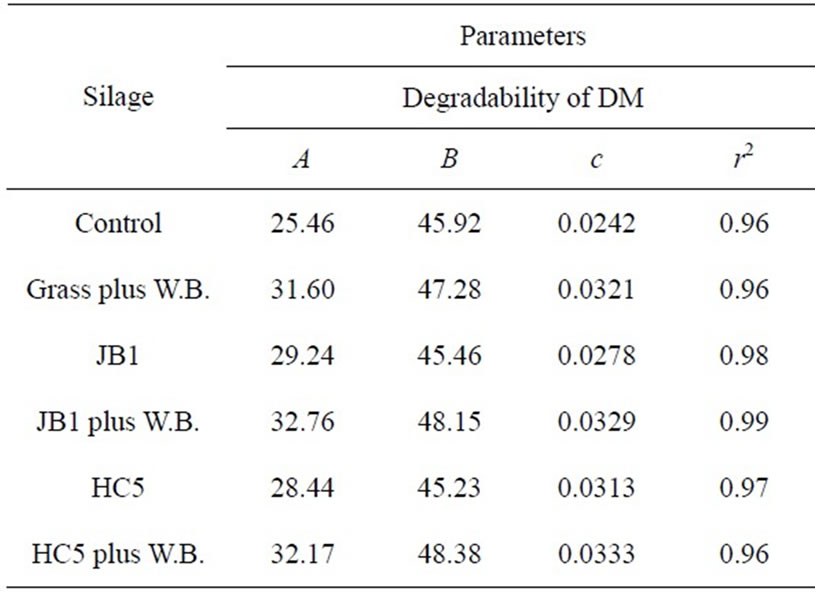
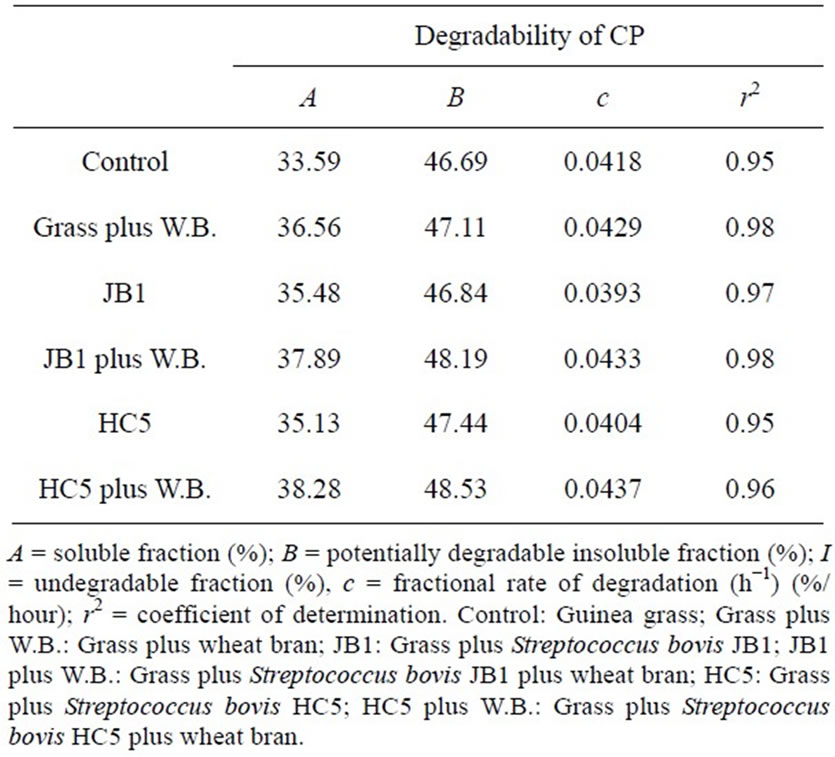
Table 3. Estimated parameters of ruminal degradability of dry matter (DM), crude protein (CP) of Guinea grass silages.
silage inoculated with Streptococcus bovis JB1 and HC5. In the study by Henriques et al. [23], it was also observed the superiority of fraction “a” of dry matter and crude protein in elephant grass silage inoculated with microbial additive. While Pires et al. [24], comparing silages of corn, sorghum and Brachiaria brizantha grass found a higher value of the fraction “a” for corn silage (38.5%) followed by sorghum silage (21.4%) and grass Brachiaria brizantha silage (12,5%). Possibly, corn silage has a higher residual sugar content, giving it a greater fraction “a” in relation to sorghum silage and grass Brachiaria brizantha silage.
According to Tonanes et al. [25] the disappearance of fraction “a” characterizes the solubilization of sugars and soluble nitrogen compounds remaining from the fermentation in the silo, consisting mainly of sucrose, fructose, glucose and small amounts of mannose and galactose.
Higher values of potentially degradable insoluble fraction “b”, of dry matter and crude protein, were observed in silages inoculated with Streptococcus bovis HC5 and JB1 with added wheat bran, as these inoculants resulted in higher values—48.38% and 48.15% for dry matter and 48.53% and 48.19% for crude protein, respectively. The degradation rate of the potentially degradable fraction of crude protein varies from 2% to 8%/hour (NRC [26]).
Evaluating different grasses silage [24] observed for the fraction “b” of 43.6%, 52.5% and 48.3% for corn silage, sorghum and Brachiaria brizantha, respectively. Ferreira et al. [12], however, despite higher values of fraction “a”, the potentially degradable insoluble fraction “b” of both the dry matter as well ascrude protein were lower in silage inoculated with Streptococcus bovis.
For the degradability of NDF and ADF, the highest values of potentially degradable insoluble fraction “b” were found in silages inoculated with microbial additive plus wheat bran, as well as for the fractional rate of degradation “c” (Table 4). The FDA degradation is closely related to the digestibility of food and, therefore, its utilization or its degradation will be higher or lower according to their composition, since the lignin present in the FDA is not used (Silva and Queiroz [16]).
For the non-degradable fraction “I” the response to the NDF and ADF was inversely proportional to the potentially degradable insoluble fraction “b”. According to Chesson et al. [27], the variation in the fraction (I) is due to natural selectivity of rumen bacteria by different types of substrates. This observation goes along with the fact that the use of microbial inoculant combined with wheat bran potentialized the silage usage, for having favored the microbial population in the rumen, thereby influencing the degradation of silages.
Another important factor is that as the levels of constituents of the fibrous fraction were influenced by microbial additives along with wheat bran, a reduction of the fiber fraction in neutral detergent fiber (Table 1) in the silo may have occurred, due to the possibility of acid hydrolysis of the hemicellulose, as a consequence of the reduction of the medium pH by the fermentation conducted by lactic acid bacteria (Muck [28]; Penteado et al. [6]), which allowed greater degradability of potentially degradable insoluble fraction “b” as seen in Table 4.
The values of potential and effective degradability of dry matter (DM), crude protein (CP), neutral detergent fiber (NDF) and acid detergent fiber (ADF) of the silages are presented in Table 5. The effective degradability was estimated considering the passage rates of 2%, 5% and 8% per hour. The measurement of degradability in the rumen, without considering the rate of passage, may overestimate the extent of degradation, because food particles are subject to the passage to the next compartment, before being completely degraded.
The values of potential degradation of dry matter and crude protein for silages inoculated with Streptococcus bovis containing added wheat bran had a similar re-
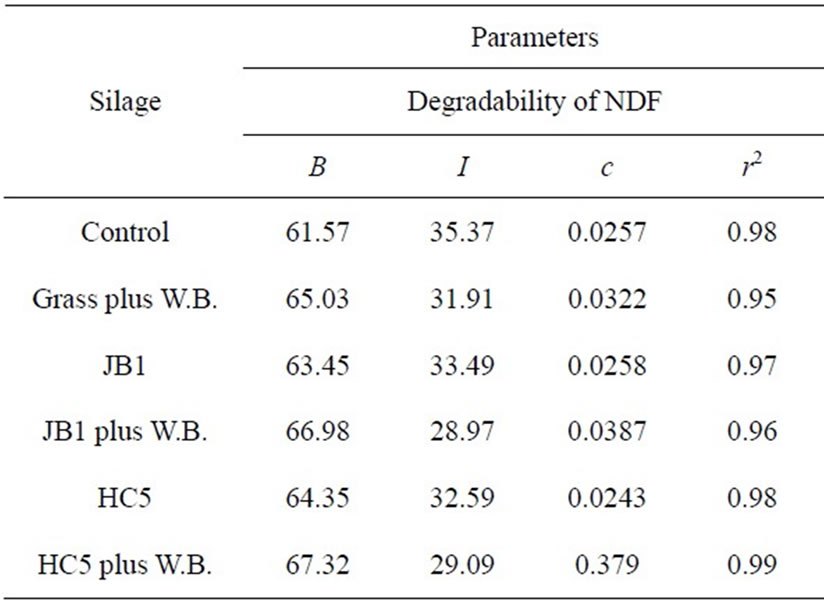
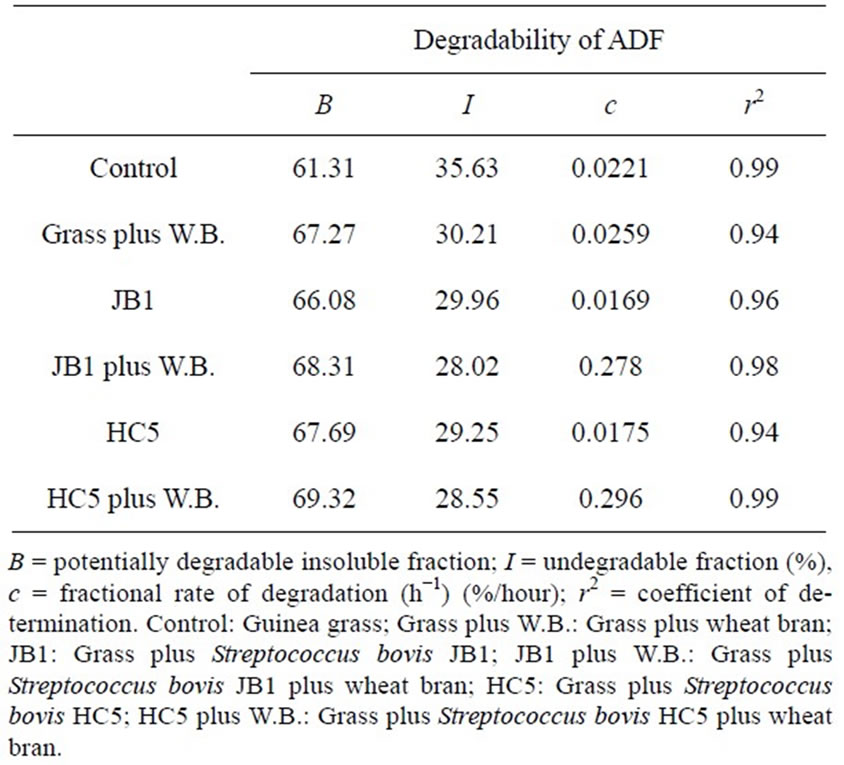
Table 4. Estimated parameters of ruminal degradability of neutral detergent fiber (NDF) and acid detergent fiber (ADF) of Guinea grass silages.
sponse, of around 78% (Table 5). Results similar to those observed for the potential degradation of neutral detergent fiber and acid detergent fiber in the course of 2.5% and 8% per hour, highlighting the potential use of Stretococcus bovis with and hygroscopic source with soluble carbohydrate in silage production.
Lower results of potential degradability of dry matter were observed by Cabral et al. [29], who assessed the degradability of elephant grass silage and found potential degradability of 64.9% of DM. The low potential degradability of dry matter reported by these authors can be attributed to the more advanced stage of maturation of elephant grass used (120 days of growth), as tropical grasses, despite their high productivity, present over their growth cycle high percentage of cell wall (NDF), a fraction of slow and incomplete digestion, which occupies much space in the gastrointestinal tract (Mertens [30]) and causes changes in digestion and affect food intake (Mertens [30]; Van Soest [31]).
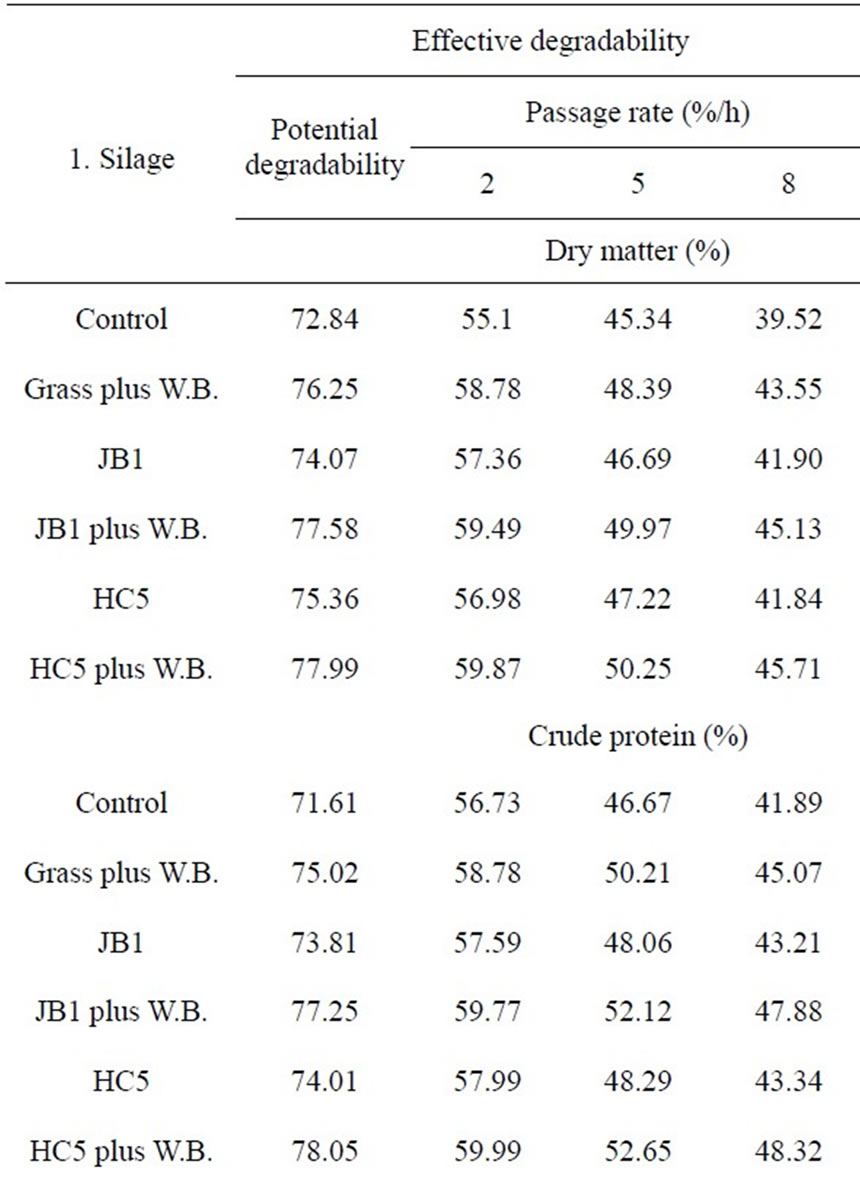
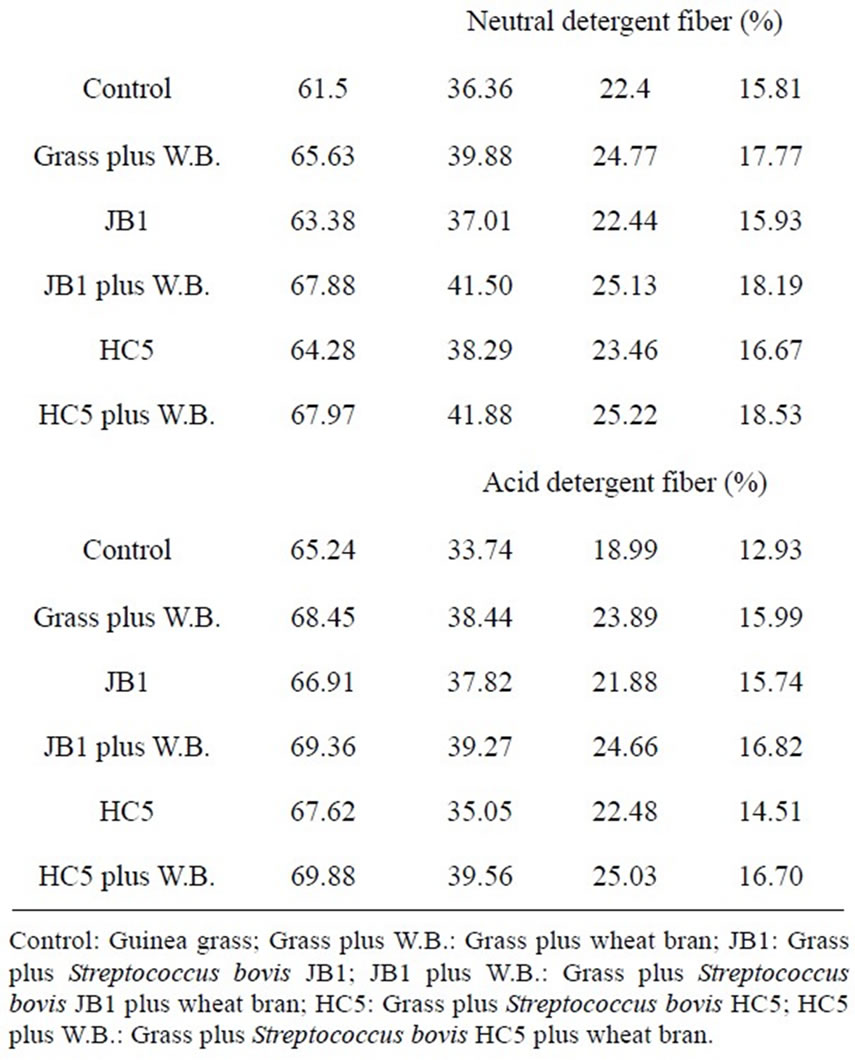
Table 5. Potential degradability (PD) and effective (ED) of dry matter (DM), crude protein (CP), neutral detergent fiber (NDF) and acid detergent fiber (ADF) of Guinea grass silages, calculated for passage rates of 2%, 5% and 8%/h.
4. CONCLUSION
The addition of 10% wheat bran together with Streptococcus bovis is effective in improving the effective degradability of dry matter, crude protein, neutral detergent fiber, acid detergent fiber of Guinea grass silage.