Different Influence of Structure Elements of Ionic Liquids on the Knoevenagel Condensation Reactions ()
1. Introduction
Condensation reactions have a major role in the transformations of organic substances. These reactions are used for syntheses of both linear and cyclic substances, including heterocyclic compounds. Condensation reactions usually require catalysts―acids or bases, as well as appropriate solvents. Condensation reactions are most commonly performed in the media of organic solvents. However, the use of these solvents involves the risks of intoxication for laboratory workers, combustion or explosion. For eliminating these risks, in the last decades, ionic liquids (ILs), as substances that are environmentally friendly and harmless for workers, have been increasingly used instead of organic solvents. Furthermore, ILs possess an outstanding ability to dissolve most organic and inorganic substances, providing homogeneous media for condensation reactions. ILs also quite often serve as catalysts for these reactions. A particular advantage is the possibility of reusing ILs several times without any purification after every application.
The use of ILs in condensation reactions has been extensively described [1] [2] [3] . Nevertheless, the influence of every structure element of an IL on its catalytic ability is not fully understood, and this does not allow the selection of the very best medium for a specific reaction, nor to choose the optimal structure of IL for a catalytic application. The role of the anion in ILs has been evaluated and described most extensively, and ILs with the acetate or other carboxylate anion have been proposed for the performance of condensation reactions most often [4] [5] [6] . Much less is known about the possible utilization of ILs with other anions for the needs of catalysis [7] . The role of hydrophilicity (or hydrophobicity) and/or specific solvation ability (hydrogen bond, etc.) has clearly been incompletely appreciated for the acceleration of organic transformations in IL media. On this account, an attempt is made in the present study to evaluate systematically the effect of most significant structure elements of ILs on the rate and yield of a commonly used condensation reaction.
2. Results and Discussion
ILs with the 1,3-disubstituted imidazolium cations (1, 2) and dimethyl phosphate and chloride anions were selected for the study because of their increased thermal and chemical stability [1] [2], the imidazolium salts with the chloride anion being used mainly for comparison. The structure of the imidazolium cation is easy to change by introducing different substituents in the aromatic ring to design the structure of IL that imparts the necessary features to the cations of ILs 1 and 2. ILs with aliphatic cation (3) but the same two anions are included in the study just for comparison.
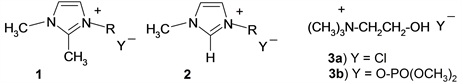
Note: DMP―dimethyl phosphate.
ILs with the DMP anion have already been successfully used in our laboratory simultaneously as reaction media and catalysts in some condensation reactions [7] [8], and they are considerably more thermally stable than ILs with the acetate or other carboxylate anions (imidazolium salts with the DMP anion decompose at temperatures ≥ 240˚C [8] ). In the present study, ILs with the DMP anion were prepared by direct alkylation of the corresponding 1-substituted imidazole with trimethyl phosphate or by metathesis of the chloride anion in 1,3-disubstituted imidazolium chlorides with the help of trimethyl phosphate, as described elsewhere [7] [8] . The obtained ILs were fully characterized by 1H NMR spectra, chromatography and determination of their moisture content and purity (content of basic substance). The 1H NMR spectrum of a most frequently used IL with the DMP anion (2d) is presented below (Figure 1), just for illustration. Resonanse signals of DMP anion protons at 3.26 and 3.23 ppm in comparison with protons at C4 and C5 in imidazolium cation at 7.81 - 7.74 ppm serve well for confirming its structure.
The DMP anion belongs to the soft anions, while the chloride anion―to the hard anions. The contrary character of both anions allows expecting different effects of ILs with these anions on the rates of the investigated condensation reaction.
The Knoevenagel condensation is one of the most significant commonly used reaction for the formation of the C = C bond in organic synthesis. The Knoevenagel condensation reaction between para-methoxybenzaldehyde (4) and ethyl cyanoacetate (5) in the investigated ILs was selected for evaluation of the influence of structural elements of various ILs on the rates of the condensation reaction. ILs served both as reaction media and as catalysts in these reactions. The reaction yields were calculated against the theoretically possible values, according to the reaction equation (see below). The methoxy group in the aromatic aldehyde slightly decreases the condensation reaction rate by comparison with unsubstituted benzaldehyde and thus facilitates determination of the rate of a reaction that is otherwise too fast.
![]()
![]()
Figure 1. 1H NMR spectrum of 1-butyl-3-methylimidazolium dimethyl phosphate (2d).
Again, the 1H NMR spectrum (Figure 2) is the most convincing proof of the structure of the condensation reaction product (6).
The influence of two lines of ILs with the same imidazolium cations and chloride or DMP anions on the condensation reaction rates was systematically compared in this paper, the cations having different substituents at the C2 and N3 atoms in the imidazolium ring (1, 2). In this way, the influence of the three most significant structure elements of ILs on transition states of the Knoevenagel condensation reaction and, consequently, the rates of these reactions, were measured:
・ influence of the type of anion;
・ influence of the hydrophobicity (accompanied by the steric effect) of the cation;
・ influence of the ability of the cation to form hydrogen bonds.
2.1. The Choice of Reaction Conditions
The starting reaction conditions were chosen for the condensation of para-methoxybenzaldehyde (4) and ethyl cyanoacetate (5) based on literature data [7] [8] and results of our preliminary experiments, namely, a reaction in 1-butyl-3-methylimidazolium dimethyl phosphate (2d) at 80˚C. In order to
![]()
Figure 2. 1H NMR spectrum of ethyl 2-cyano-3-(4-methoxyphenyl)propenoate (6).
determine the necessary duration of the reaction, samples were taken from the reaction mixture in certain time intervals. Every sample was extracted with the mixture of ethyl acetate and saturated NaCl water solution (1:1) several times, the organic layer was separated, and the condensation reaction product―ethyl 2-cyano-3-(4-methoxyphenyl)propenoate (6)―in the joint organic layer was immediately analyzed by gas chromatography (GC). The obtained results are presented in Figure 3.
The performed GC measurements show that the investigated condensation reaction proceeded at 80˚C, reaching the highest yield (94%) as soon as in 15 minutes (Figure 3). The yield did not change afterwards, and, therefore, a 15-minute time span was adopted as sufficient for the reaction to complete in all other ILs at 80˚C, with a molar ratio of 4:5:IL = 1:1:1.
2.2. The Role of the Anion
In order to evaluate the influence of the anion of ILs on condensation reaction rates, 16 structurally different ILs with the DMP and chloride anions were compared in the given condensation reaction. The obtained yields of the isolated product 6 are shown in Figure 4. No product 6 was isolated in experiments in the ionic liquid containing 2-hydroxyethyl group in their cation and chloride anion [MEtOHIm] [Cl] (2i), in contrast to IL with the same cation and DMP anion (2j).
Yields of the condensation product 6 were always higher in all ILs with the DMP anion than in the corresponding ILs with the chloride anion. The latter does not show any basicity in water solutions. However, the Kamlet-Taft β parameter of the investigated ILs with the chloride anion (1.13) was quite close to the same parameter of IL with the acetate anion (1.18) [9], the last showing an efficient catalytic activity in many Knoevenagel condensation reactions [7] . On this account, our experiments suggest that the chloride anion is likely to provide some basicity in IL media, at least in some of them. The isolation of the condensation reaction product 6 was not successful in the IL [MEtOHIm] [Cl] (2i), most likely due to the effect of other structure elements of IL that will be discussed further. Our results convincingly prove the superiority of ILs with the DMP anion in comparison to those with the chloride anion (Figure 4). These results do not come as a surprise, because DMP is a week base even in a water medium. Even if no data about basicity of the DMP anion in ILs has been reported, our results do suggest this. At the same time, differences in the yields of the condensation reaction product 6 strongly confirm minor accelerating or decelerating effects of other structure elements in IL cations.
In order to reinforce the hypothesis about the leading effect of the anion, kinetic curves were registered for the condensation reaction in three only slightly different ILs: [BMIm] [DMP] (2d), [BMIm] [Cl] (2c), and [BMMIm] [Cl] (1c) (Figure 5).
Using the same cation in two of the selected ILs allows better evaluation of the effect of different anions (Figure 5). These kinetic curves confirm once more the fact noticed earlier that ILs with the DMP anion better catalyze the condensation reaction than those with the chloride anion; yields of the product 6, determined by GC, reached 97% in case of the DMP anion against an 84% yield with the chloride anion. The comparison of other ILs demonstrated different divergences, due to various substituents in the imidazolium cations (Table 1). Nevertheless, the trend remained the same: ILs with the DMP anion always provided higher yields of the isolated product than those with the chloride anion.
2.3. The Influence of the Substituent at the C2 Atom in the Imidazolium Cation
The third kinetic curve, which has not been considered above, is included in Figure 5. It is the curve for the reaction in [BMMIm] [Cl] (1c) that would allow to appreciate the minor negative influence of the substituent at the C2 atom (methyl group) in the imidazolium ring on the reaction course. Yields of the isolated product 6 showed a similar tendency in ILs with both the DMP and the chloride anions: the presence of a methyl group always slightly delayed the reaction and, consequently, decreased the yields (Table 2).
![]()
Figure 3. Yield of 2-cyano-3-(4-methoxyphenyl)propenoate (6) in time (in the medium of [BMIm] [DMP] at 80˚C, with a molar ratio of 4:5:IL = 1:1:1, determined by GC).
![]()
Figure 4. Isolated yields of 2-cyano-3-(4-methoxyphenyl)propenoate (6) in different ILs (after 15 min at 80˚C, with a molar ratio of 4:5:IL = 1:1:1).
![]()
Figure 5. Yield of 2-cyano-3-(4-methoxyphenyl)propenoate (6) in time in slightly different ILs (at 80˚C, with a molar ratio of 4:5:IL = 1:1:1, determined by GC).
![]()
Table 1. The effect of the type of anion on the yield of the condensation reaction product.
* Yield of the isolated product (6) after 15 minutes at 80˚C.
![]()
Table 2. The effect of the substituent at the C2 atom in the imidazolium cation on the yield of the condensation reaction product.
* Yield of the isolated product (6) after 15 minutes at 80˚C.
The C2-H bond in the imidazolium cations possess a property of a weak C-H acid. Consequently, it is able to form hydrogen bonds between the transition state/states of the condensation reaction and the IL anions, which might be the explanation of the observed facts. The mentioned hydrogen bond might be stronger with the chloride anion (hard base) than with the DMP anion (weak base). As a result, the hard base (chloride anion) decreases the otherwise beneficial effect of the C2-H bond on the transition state/states and lowers the yield of the condensation reaction.
2.4. The Influence of the Length of the Linear Alkyl Chain at the N3 Atom in the Imidazolium Cation
The hydrophilicity of ILs decreases with the increasing length of linear alkyl substituents in their imidazolium cations. Furthermore, a sufficiently long side chain of the cation can coil up into a globe, take a position in the space around the cation, or interfere in some other way with the rate determining the transition state of the condensation reaction and thereby hamper the rate-limiting step of the reaction. Besides, sufficiently long linear alkyl chains in the cation (the octyl and dodecyl groups at the N3 atom in the IL cation) can provide ILs with properties of surfactants with the following formation of micelles. In our experiments, the formation of micelles (and, possibly, their steric effects) were clearly observed only in ILs with the chloride anion and the C2-H bond in their cations (Table 3).
A small opposite effect was observed when a methyl group was attached to the C2-atom in the IL cation―the yield slightly decreased with the increase in the length of the alkyl chain. This means that the C2-H group in the cation slows down the condensation reaction rate. The micellar effect also did not appear in ILs with a methyl group at the C2-atom, because the chloride ions in these ILs have no sufficiently acidic hydrogen atom to form hydrogen bonds. At the same time, hydrogen bonds appear if ILs have a C2-H bond. Furthermore, as the hydrogen bond is directly responsible for the decrease in the basicity of the chloride anion, the basicity becomes dependent on the substituent (CH3 or H) at the C2-atom in the imidazolium cation. The observed facts allow to put forward a hypothesis that the length of a side chain in imidazolium chlorides slows down the condensation reaction rate only in case if the hard chloride anion is capable to form hydrogen bonds. These facts also confirm the more important earlier observation that the anion type has a considerably higher influence on the transition state of condensation reactions than other structure elements of IL cations. Admittedly, this is only a hypothesis made from observations of just one reaction, therefore requiring further investigation.
Data presented in Figure 4 and Table 3 confirm that the substituent at the C2 atom in the imidazolium cation affects the condensation reaction rates in a lesser extent in ILs with the DMP anion than in those with the chloride anion. Even more, it is difficult to notice any certain trend in ILs with the DMP anion. Most likely, DMP, being a soft anion, is able to form a weaker hydrogen bond, if any, with the C2-H bond than the hard chloride ion.
The abovementioned micelle formation during the condensation reaction was observed in our experiments when the products were extracted with the mixture of EtOAc and water. Small beads of ILs in water formed in the interlayer between the solvents during the extraction process from ILs with the dodecyl and octyl groups at the N3 atom in IL cations, and it was quite difficult to separate these beads from the EtOAc solution of the product.
In order to ascertain the statement about the effect of the length of the side chain, three kinetic curves were once again registered for the condensation reaction in ILs with the same DMP anion but different substituted imidazolium cations, namely, [BMIm] [DMP] (2d), [MOIm] [DMP] (2f), and [DDMIm] [DMP] (2h) (Figure 6).
![]()
Table 3. The influence of the length of the linear alkyl chain at the N3 atom and the substituent at the C2 atom in the imidazolium cation on the yield of the condensation reaction product.
* Yield of the isolated product (6) after 15 minutes at 80˚C.
![]()
Table 4. The influence of the possible hydrogen bond formation between the imidazolium cation and the transition state of the condensation reaction*.
* Yield of the isolated product (6) after 15 minutes at 80˚C; ** yield determined by GC.
![]()
Figure 6. Yield of 2-cyano-3-(4-methoxyphenyl)propenoate (6) in time in ILs with the same DMP anion and different cations (at 80˚C, with a molar ratio of 4:5:IL = 1:1:1, determined by GC).
The almost identical kinetic curves also indicate that, in contrast to the influence of the anion (e.g., DMP), the linear alkyl substituents in the cation structures of ILs have only a minor effect on the condensation reaction rates. Yields of the product (6) are high (93% - 97%) in all three reactions, and only an insignificant decrease in the yield can be observed in ILs with the lengthening of the substituent chains in their cations (Figure 6).
2.5. The Influence of the Possible Formation of a Hydrogen Bond in the Imidazolium Cation
If the IL cation contains a hydroxyl group, the latter can form stronger hydrogen bonds with the transition state of the condensation reaction than the C2-H group. The data presented in Figure 4 and Table 4 evidence that the presence of OH groups has a clearly negative influence on the condensation reaction rates (and yields). The harmful effect increases in ILs containing the hard chloride ion instead of the soft DMP anion. A drastic decrease in the yield of the product (6) was observed, and sometimes no product could be isolated in the media of ILs with the chloride anion, such as [HOEtMIm] [Cl] (2i), even if a negligible yield (7%) could also be detected by GC in this case. A similar situation can be observed in reactions in the media of choline chloride (3a) and choline dimethyl phosphate (3b).
The introduction of a hydroxyl group in the imidazolium cation makes the reaction medium partly protic, similar to water or protic solvents. The chloride ion definitely has no measurable basicity in such media and, correspondingly, no capacity to convert ethyl cyanoacetate into the corresponding anion in order to start the catalytic condensation reaction. Further condensation process is not imaginable without this step.
Hence, ILs―imidazolium salts with the DMP anion can be considered as useful media and catalysts at the same time for the Knoevenagel condensation reaction between para-methoxybenzaldehyde and ethyl cyanoacetate and between aromatic aldehydes and activated methylene compounds in general. The influence of the type of anion on rates and yields of the condensation reaction is much more significant than the effects of other structural elements of ILs.
3. Experimental
Chemical reagents of high purity were obtained commercially from Alfa Aser or Sigma Aldrich and were used without further purification. 1H NMR spectra were recorded in deuterated solvents with a Bruker Fourier 300 at 300 MHz, using the solvent as the internal standard. Moisture (water content) in ILs before their use was analyzed with a Karl Fisher 836 Titrant Metrohm automatized titrator. Hydranal-Composite 5 (Riede de Haën®) one-component titrant (reagent) was used, the highest systematic error of the analysis being ±0.2%. Melting points were registered with the Stuart SMP3 instrument (accuracy ± 0.1˚C). Purity of ILs was measured by titration: 1) for ILs with the DMP anion―with perchloric acid in glacial acetic acid using Solvotrode (Metrohm AG 9101 Herisau); 2) for ILs with the chloride anion―with silver nitrate in water solution. Gas chromatography (GC) was performed using a YL GC-6100 instrument with an EquityTM-5 (30 m × 0.25 mm × 0.25 mm) flame ionization detector and column, the mobile phase being helium with a constant flow rate of 1.0 mL/min. Gas chromatography-mass spectrometry (GC-MS) was performed using a Shimadzu GCMS-QP2010 instrument with an Agilent DB-5 MS capillary column (25 m, internal diameter 200 µm, layer thickness of the liquid phase―0.2 µm, the mobile phase being helium with a flow rate of 1.1 mL/min at the pressure of 40.7 kPa).
3.1. Synthesis of Ionic Liquids
ILs with the DMP anion used for investigation were synthesized in two ways: 1) ILs with the chloride anion were first prepared by the traditional alkylation reaction of 1-substituted imidazoles with the corresponding alkyl chloride, followed by the chloride ion metathesis reaction into DMP with trimethyl phosphate (route A); 2) direct alkylation of 1-substituted imidazole with trimethyl phosphate (route B), as described earlier [7] [8] .
1-Butyl-2,3-dimethylimidazolium chloride (1c). A typical experiment. 1,2-dimethylimidazole (9.61 g; 0.10 mol), 1-chlorobutane (12.03 g; 0.13 mol), magnetic stirrer, and ethyl acetate (6 mL) were placed in a sealed screw-top steel pressure tube. The tube was placed in a glycerol bath and stirred at 80˚C for 72 hours. After cooling to room temperature, the reaction mixture was poured into a round-bottom flask and put in a freezer for 24 hours. The obtained crystalline mass was filtered, washed with ethyl acetate (4 × 25 mL) on the filter, then dried under vacuum at 40˚C and, after that, under high vacuum (0.5 mbar) at 60˚C for 6 hours. IL (1c; 23.34 g; 89%) was obtained as a white crystalline substance with m.p. 93˚C - 94˚C. 1H NMR spectrum (300 MHz, DMSO-d6, δ): 7.63 (2H, s, NCH = CHN); 4.11 (2H, t, NCH2-CH2-CH2-CH3); 3.74 (3H, s, CH3NCCH3); 2.58 (3H, s, CH3NCCH3); 1.68 (2H, m, NCH2-CH2-CH2-CH3); 1.29 (2H, m, NCH2-CH2-CH2-CH3); 0.92 (3H, t, NCH2-CH2-CH2-CH3) ppm.
Other ILs with the chloride ion were obtained in a similar way.
1-Butyl-2,3-dimethylimidazolium dimethyl phosphate (1d) (route A, obtained by the metathesis of the chloride anion). A typical experiment. 1-Butyl-2,3-dimethylimidazolium chloride (18.67 g; 10.0 mmol) and trimethyl phosphate (35.02 g; 25.0 mmol) were placed in a 50 mL round-bottomed flask equipped with a reflux condenser and a CaCl2 drying tube. The obtained mixture was stirred at 110˚C for 24 h. Toluene (5 × 10 mL) was added to the crude product, and the mixture was vigorously stirred and heated to reflux for 10 minutes. The toluene layer was then decanted while hot. The procedure was repeated four more times. Any remaining solvent was removed by vacuum evaporation (10 mbar, 70˚C, 4 h). The pure product was dried under high vacuum (0.5 mbar, 70˚C, 8 h) and was subject to AgNO3 analysis to confirm the absence of a starting material. Dimethyl phosphate (1d; 24.13 g; 92%) was obtained as an oil that solidifies into a white crystalline substance with m.p. 92˚C - 93˚C within 24 h in a refrigerator. 1H NMR spectrum (300 MHz, DMSO-d6, δ): 7.64 (2H, d, NCH = CHN); 4.13 (2H, t, NCH2-CH2-CH2-CH3); 3.74 (3H, s, CH3NCCH3); 3.23 (6H, d, P(OCH3)2); 2.58 (3H, s, CH3NCCH3); 1.67 (2H, m, NCH2-CH2-CH2-CH3); 1.29 (2H, m, NCH2-CH2-CH2-CH3); 0.92 (3H, t, NCH2-CH2-CH2-CH3) ppm.
Other ILs with the dimethyl phosphate anion were obtained in a similar way.
1,2,3-Trimethylimidazolium dimethyl phosphate (1b) (route B, obtained by direct alkylation with trimethyl phosphate). A typical experiment. Trimethyl phosphate (8.41 g; 0.06 mol) was added dropwise to 1,2-dimethylimidazole (4.81 g; 0.05 mol) with vigorous stirring in a round-bottom flask. The mixture was stirred for 1 h at room temperature, and then the temperature was raised to 80˚C. 10 mL of acetonitrile was added after 30 minutes, and the content of the flask was stirred at 80˚C for 48 hours. The hot solution was poured into a conical flask and left at room temperature for 24 hours. The formed precipitate was filtered, washed with ethyl acetate on the filter and then dried in vacuum (0.5 mbar) for 6 hours. IL (1b; 12.66 g; 91%) was a white crystalline substance with m.p. 124˚C - 126˚C. 1H NMR spectrum: (300 MHz, DMSO-d6, δ): 7.59 (2H, s, NCH = CHN); 3.75 (6H, s, CH3N-C(CH3) = NCH3); 3.23 (6H, d, P(OCH3)2); 2.55 (3H, s, NC(CH3)N) ppm.
Other ILs with the dimethyl phosphate anion were obtained in a similar way.
3.2. Quantitative Analyses of ILs with the Dimethyl Phosphate Anion
A sample of an ionic liquid with the DMP anion (1, 2, or 3b) (~100 mg) was dissolved in glacial acetic acid (50 mL) and titrated with the solution of perchloric acid in glacial acetic acid (0.05 mol/L) in the equipment for potentiometric titration. The purity (content of the main substance, %) of the sample was calculated from the obtained titration curves.
3.3. Condensation Reaction in IL Media
Ethyl 2-cyano-3-(4-methoxyphenyl)-2-propenoate (6). Ethyl cyanoacetate (0.57 g, 5 mmol) was added to the solution of 4-methoxybenzaldehyde (0.68 g, 5 mmol) in 1-butyl-3-methylimidazolium dimethyl phosphate (1.32 g, 5 mmol), and the reaction mixture was stirred at 80˚C for 15 minutes. Distilled water (3 mL) was added to the obtained yellow reaction mixture and the stirring was continued for another 15 minutes. The mixture was extracted with ethyl acetate (7 × 7 mL), and the joint extract was dried over MgSO4 for 16 h. The filtered solution was evaporated in vacuum, and the obtained yellow substance was crystallized from ethanol. Yellow crystalline ethyl 2-cyano-3-(4-methoxyphenyl)-2-propenoate (1.03 g, 89%) was obtained with m.p. 83˚C - 84˚C (lit. [10] m.p. 83˚C). 1H NMR spectrum (300 MHz, CDCl3, δ): 8.19 (1H, s, C-CH-C-CN), 8.03 (2H, d, CHarom), 7.02 (2H, d, CHarom), 4.37 (2H, m, COO-CH2-CH3), 3.91 (3H, s, O-CH3), 1.41 (3H, t, COO-CH2-CH3) ppm.
Condensation reactions in other ILs were performed in a similar way, and the obtained yields of the product (6) were recorded in Tables 1-4.
4. Conclusion
ILs with the DMP anion are appropriate reaction media and catalysts at the same time for the Knoevenagel condensation reactions to provide high yields of the condensation product. ILs with the chloride anion also show weaker catalytic properties in these reactions. Anions are the most significant structure elements in the ILs used in the mentioned syntheses. The length of IL cation side chains has a negligible effect on the performance of ILs, whereas the possibility of formation of hydrogen bonds between ILs and transition states of the reaction demonstrates a clearly negative reaction rate-reducing effect.
Acknowledgements
We gratefully acknowledge the Research Foundation of the University of Latvia for the financial support of this study.