Biodegradability of Formulated Enzymatic Solution: An Alternative Waste Reduction System ()
1. Introduction
Cabanatuan City is considered as the economic heart of Nueva Ecija and is the focus of a metropolitan area of more than 300,000 people. Cabanatuan City residents, industrial and business establishments generated tons of garbage every day. Around 50% of which is biodegradable 35% recyclable and 15% residual. To address the growing problem of waste disposal, the City Government of Cabanatuan instituted ways of improving its existing dumpsites and transfer facility stations.
The former dumpsite in Valle Cruz, Cabanatuan City was closed to give way to its rehabilitation aimed at removing odorous pollution that is affecting the residents within and around the vicinity. These pollutants are mostly organic solid wastes, which have the highest percentage amount based on the previous waste analysis characterization system. These solid wastes are non-soluble materials that were discarded in a solid or semi-solid form. The semi-solid form of waste has the quality of both solid and liquid- like sludge.
Geographically, Valle Cruz dumpsite is a residential and agricultural area where emission of odorous pollutants poses grave effects on the health of the residents. Extension of the said facility to accommodate extra tons of wastes costs large amount of taxpayer’s money and land conversion causes social and economic nuances. A more viable solution is the use of alternative means of waste reduction and anti-pollution technology, where the application of enzymatic solutions can be of great advantage. Extracellular enzymatic solutions can hydrolyze and digest insoluble organic waste [1] .
Enzymatic solutions are natural, nontoxic substances that use enzymes that help the bacteria to digest organic materials directly. Enzymatic solutions are formulated specifically to dispose of soils safely, economically and rapidly [2] [3] [4] . They contain the necessary quality and quantity of specific enzyme systems, both aerobic and anaerobic bacteria, and microbial nutrient. Enzymatic solution works quickly around 2 weeks [5] and efficiently in the digestion of chemical and organic waste with no odor or noxious gas emissions. The micro-organism and right type of enzymes break the waste apart and the bacteria itself converts this waste into two basic compounds: carbon dioxide and water as indicated by the C/N ratio [6] . This resulted in faster degradation of wastes with minimal residual component, and the absence of foul odors [7] [8] [9] , thus making it a great candidate for obtaining waste disposal method that is environmentally and economically viable [10] . Moreover, the uses of enzyme solutions make the composting process of biodegradation of organic waste as self sustaining due to the soluble organic matter that forms and act as fuel for microorganisms for further degradation [11] . Aquatic organisms such as fish have a vast array of diversified genetic material that makes it a great source of different enzymes [12] .
This study aimed to develop formulated enzymatic solution (FES) from fish intestines and fruit peels that could aid in biodegradation of organic wastes comparable with commercial enzymatic solutions based on the amount of CO2 evolved by and the weight loss of the organic wastes treated with different developed FES from fish intestines and fruit peels and compare the biodegradation efficiency of the best formulated enzymatic solution with that of commercial enzymatic solution based on the amount of CO2 evolved by organic wastes, weight decrease of organic wastes and result of odor survey test. Lastly, it aimed to determine the biodegradability, Dt, of the formulated enzymatic solution.
2. Methodology
2.1. Experimental
The true experimental method of research using CRD- pre-test-post-test design [13] was used in conducting the study.
In preparing the commercial enzymatic solution, 41.70 ml of commercial enzymatic solution was diluted in 1.25 liters of distilled water based on the product label.
Equal grams of mango and banana peels where mixed and from that mixture, 321 g of waste was taken and mixed with 107 ml molasses and 1 L of water in a 2 L container and then fermented for 3 months for the experimental enzymatic solutions. After the fermentation period, the solution was filtered and analyzed for the corresponding parameters; pH and BOD (biological oxygen demand). After the laboratory analysis dilution was adjusted to pH 7, about 8 ml of the solution was diluted to 1.9 liters distilled water. Same procedure was also applied with the use of fish intestines as a substitute of fruit peels to make enzymatic solution with the dilution of 2 ml solution in 1.2 L of distilled water.
The prepared enzymatic solutions (experimental) made from fruit peels and fish intestines were mixed to determine the optimal mixture that would produce better result in bio-decomposition and odor reduction of organic solid waste. There were 5 formulations prepared: Formulation 1, a mixture of fruit peels (25%) and fish intestines (75%); formulation 2, a mixture of fish intestines (25%) and fruit peels (75%); formulation 3, a mixture of fish intestines (50%) and fruit peels (50%); formulation 4, an enzymatic solution composed of fruit peels (100%); formulation 5, an enzymatic solution composed of fish intestines (100%).
The amount of carbon dioxide evolved was determined by gravimetric analysis. From the air compressor, a rubber hose was connected to the carbon dioxide trap which contains soda lime to remove the carbon dioxide from the continuous flow of air (Figure 1). A humidifier was used to give enough moisture responsible for the degradation of organic waste. The suction flask/pet bottle that contains water was connected to the 1L suction flask/pet bottle containing 200g of organic waste and then connected to the dehumidifying trap which contains silica gel to remove the air moisture before going to the carbon dioxide absorbing column to avoid the increased in weight of carbon dioxide trap by moisture increase. The dehumidifying trap was connected to the CO2 absorbing column that contains 80 ml of soda lime and soda talc mixture. This served as the absorbent of the carbon dioxide released by the bottle containing organic waste. The CO2 absorbing columns were weighed every 24 hours of aeration, the air flow was set to 14 L/hour [14] .
![]()
Figure 1. Set-up for determination of evolved carbon dioxide.
The mass of the evaporating dish was recorded, followed by the grinding of the test specimen using a laboratory blender. The specimen was added to the evaporating dish and weighted. After recording the mass of the evaporating dish with the test specimen, it was then oven-dried uncovered for at least 6 h at 250˚C until there was no change in mass of the sample in excess of 1 hour. The test specimen was removed at the oven and placed in a desiccator for 5 minutes taking down the mass of the oven-dried test specimen. The percentage weight loss was calculated by subtracting the initial weight initial weight from the final dry weight taken from the moisture content determination result over initial weight multiplied by 100 percent.
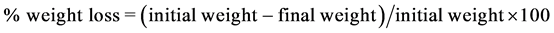 (1)
(1)
2.2. Odor Survey
During the test, the 20 panelists sniffed the prepared samples starting from the sample (a) to sample (g) absorbed via cotton balls. The arrangement was not revealed to the panelist to avoid bias. The panelists were ask to rate the samples based on the odor intensity referencing scale standard practice (Table 1). The result from the survey test was tallied to determine the difference of the odor intensity of the different samples [15] .
3. Biodegradability Determination
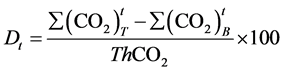 (2)
(2)
where:
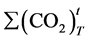 is the cumulative amount of carbon dioxide, in grams, evolved in test vessel between the start of the test and time;
is the cumulative amount of carbon dioxide, in grams, evolved in test vessel between the start of the test and time;
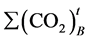 is the mean cumulative amount of carbon dioxide, in grams, evolved in the blank vessel between the start of the test and time t; and
is the mean cumulative amount of carbon dioxide, in grams, evolved in the blank vessel between the start of the test and time t; and
ThCO2 is the theoretical amount of carbon dioxide, in grams, evolved by test material [16] .
A stop gap approach for composting in solid waste estimation based on a formula developed in the 1950s and at A5TM D2974-87 Standard Test Methods for Moisture, Ash, and OM of Peat and Other Organic Soils was utilized to
![]()
Table 1. Odor intensity referencing scale standard practice.
determine the carbon content as indicated by [17] .
For the moisture determination, the mass of the evaporating dish was recorded, followed by the grinding of the test specimen using a laboratory blender. The specimen was added to the evaporating dish and weighted. After recording the mass of the evaporating dish with the test specimen it was then oven dried uncovered for at least 6 h at 250˚C until there was no change in mass of the sample in excess of 1 hour. The test specimen was removed from the oven and placed in desiccators for 5 minutes.
The mass of the covered evaporating dish was recorded, and then the oven- dried test specimen was placed in the dish taking into consideration the mass of the dish and specimen. The cover was removed and placed the dish into the muffle furnace. Gradually, the temperature in the furnace was set to 440˚C and held until the specimen has no change of mass after a further period of heating. Afterwards, the specimen was cooled into the desiccators and then recorded the final mass. This was done in order to determine the ash content.
4. Results and Discussion
4.1. Amount of CO2 Evolved by Organic Waste per Day Using Different Formulated Enzymatic Solutions
Fish intestines contain enzymes that facilitate digestion, absorption and assimilation of molecules like proteins and lipids [18] [19] [20] [21] . Thus it can degrade organic waste and transform it into carbon dioxide and water. Organic waste that contains dried leaves was enclosed in plant cell walls that make it hard to degrade causing long time degradation. Fruit peels contain plant cell wall degrading enzymes that aid in the degradation process. Fruit peels usually contain xylanase, polygalacturonase, cellulase and α-amylase [22] [23] [24] [25] . In this study, amount of carbon dioxide evolved per day was determined for the different enzyme solutions formulated. As shown in Figure 2, the formulation 3 (F3) (50% fruit peels and 50% fish intestines) has the highest increased amount of evolved CO2 per day with about 1.96 g in 7 days and reached its peak (0.5 g) on day 2. However, on day 5 it falls gradually into 0.4 g and 0.26 g at day 7, respectively. It can also be noted that among the developed FES, F3 reached the greatest amount of CO2 evolved within the least number of elapsed time.
![]()
Figure 2. Amount of carbon dioxide evolved/day.
4.2. Weight Decrease of Organic Waste per Day of the Formulated Enzymatic Solutions
The organic waste sample applied with F3 (50% fruit peels, 50% fish intestines) has the greatest decreased in weight from day 0 to day 7 as shown in Figure 3. Since F3 yielded the highest amount of CO2 within the least number of days and the one with the greatest decrease in weight throughout the treatment, it was considered as the best formulation. Thus, the biodegradation efficiency of F3 was compared with that of the commercial enzymatic solution throughout the study.
4.3. Biodegradability Efficiency
The treatment of organic waste with F3 resulted to a percentage weight loss of 98.45% in 7 days which was slightly lower than the organic waste sample treated with the commercial enzymatic solution. To support the comparison, statistical analysis was done. The t-test results of total evolved CO2 and percentage weight loss which indicates that there was no significant difference between the commercial solution and F3 since the computed t-stat value is lower than the critical value. Thus, it can be noted that the biodegradability efficiency of the F3 is comparable with the commercial enzymatic solution in terms of these parameters.
The organic waste sample applied with F3 has a percentage biodegradability of 0.71 with about 505.75 milligram per kilogram per day. Organic wastes on the average has a percent biodegradability of 0.1 - 0.7. This attainment of maximum percent biodegradability of the organic waste sample may be attributed to the treatment made.
4.4. Odor Survey Result
The organic waste sample applied with the commercial enzyme solution has a
![]()
Figure 3. Decrease in weight of organic waste per day.
weighted mean of 2.7 which falls under normal odor. Commercial enzyme solution contains microbes which produces enzyme essential for odor reduction of organic solid waste. On the other hand, F3 has a weighted mean of 2.8 that also falls under the same description as having normal odor. Fruit peels contain enzymes that exhibit inhibition against gram positive and gram negative bacteria [26] [27] [28] [29] . Proliferation of these bacteria is the one responsible for the foul odor of organic waste. Thus, the application of enzymatic solution formulated resulted in normal acceptable odor of organic waste.
The t test results of odor survey indicate that there was no significant difference between the commercial enzymatic solution and F3 since the t stat is lower than the t critical value. Thus, it can be noted that the use of the developed FES comprising of fruit peels and fish intestines was comparable with the commercial enzymatic solution in terms of odor reduction upon degradation of organic waste.
5. Conclusion
Enzymatic solutions formulated from fish intestines and fruit peels may be developed to assist in biodegradation of organic wastes. Formulated enzymatic solution made from 50% fruit peels and 50% fish intestines, F3, is comparable with the commercial enzymatic solution based on the amount of evolved CO2, weight loss of organic sample and odor survey test as supported by statistical analysis. The organic waste sample applied with the most effective formulation has a percentage biodegradability of 0.71 with about 505.75 milligram per kilogram per day. This maximized percent biodegradability may be attributed to the treatment of the formulated enzymatic solution. Thus the enzymatic solution formulated can maximize the decomposition and reduce the release of unpleasant odor of organic waste comparable with commercial enzyme solution.
Acknowledgements
The authors wish to thank the NEUST Administration, faculty and staff especially those under the College of Arts and Sciences for their support.